Method for determining content of caulis spatholobi in Kangfuling capsule
A method of determination, the technology of Kangfuling gum, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems that the quality of Kangfuling capsules cannot be well controlled, and no scientific reports have been seen, so as to improve the quality inspection standard , good peak shape and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
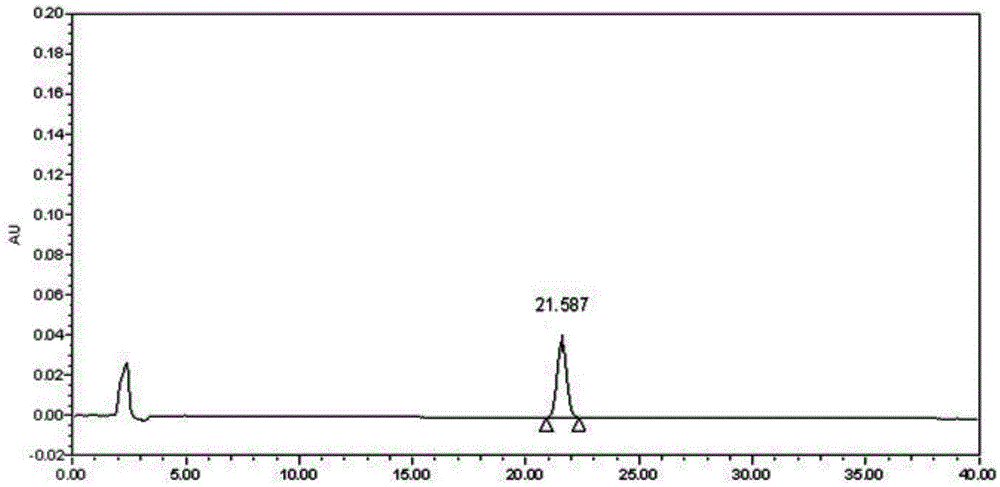
[0061] Example 1 Determination of the content of Caulis spatholobi in Kangfuling Capsules: According to Appendix VID of the 2010 edition of the Chinese Pharmacopoeia, determination:
[0062] Chromatographic conditions and system adaptability test: octadecylsilane bonded silica gel is used as a filler, acetonitrile-water=35:65 is used as the mobile phase, and the detection wavelength is 254nm;
[0063] Preparation of reference substance solution: take formononetin reference substance, dissolve it in 80% methanol, and make a solution containing 0.03mg formononetin reference substance per 1 mL as a reference substance solution;
[0064] Preparation of the test solution: Take 5g of the contents under the difference in the amount of this preparation, add 50mL of 80% methanol, and extract with reflux in a water bath for 30 minutes, filter, evaporate the filtrate to dryness, add 10mL of 80% methanol to the residue to dissolve, filter Once, take the continued filtrate as the test solution;
[00
Example Embodiment
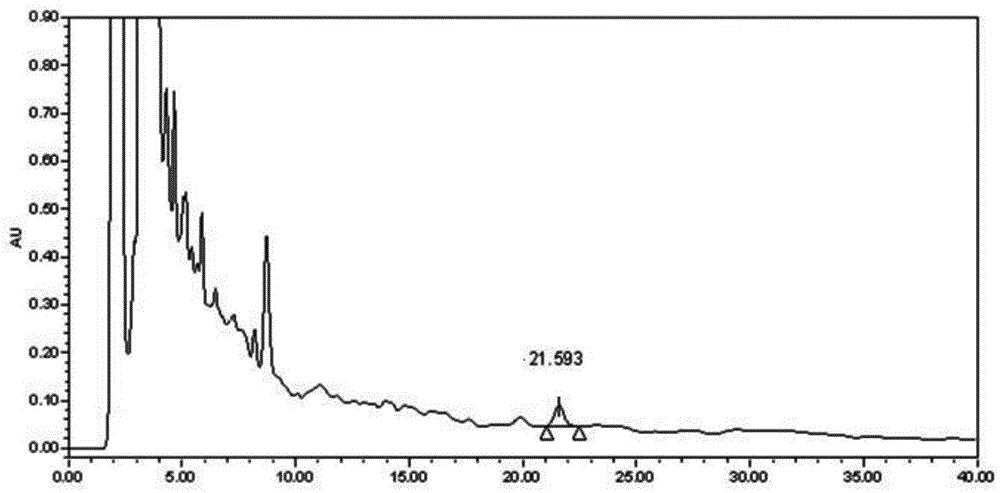
[0066] Example 2 Determination of the content of Caulis spatholobi in Kangfuling Capsules: According to Appendix VID of the 2010 edition of the Chinese Pharmacopoeia, determination:
[0067] Chromatographic conditions and system adaptability test: octadecylsilane bonded silica gel is used as filler, acetonitrile-water=40:70 is used as mobile phase, and the detection wavelength is 250nm;
[0068] Preparation of reference substance solution: take formononetin reference substance, dissolve it in 80% methanol, and make a solution containing 0.03mg formononetin reference substance per 1 mL as a reference substance solution;
[0069] Preparation of the test solution: Take 4g of the contents under the item of difference in the dosage of this preparation, add 45mL of 80% methanol, and extract with reflux in a water bath for 50 minutes, filter, and evaporate the filtrate to dryness. Once, take the continued filtrate as the test solution;
[0070] Determination: Precisely draw 15μL each of the ref
Example Embodiment
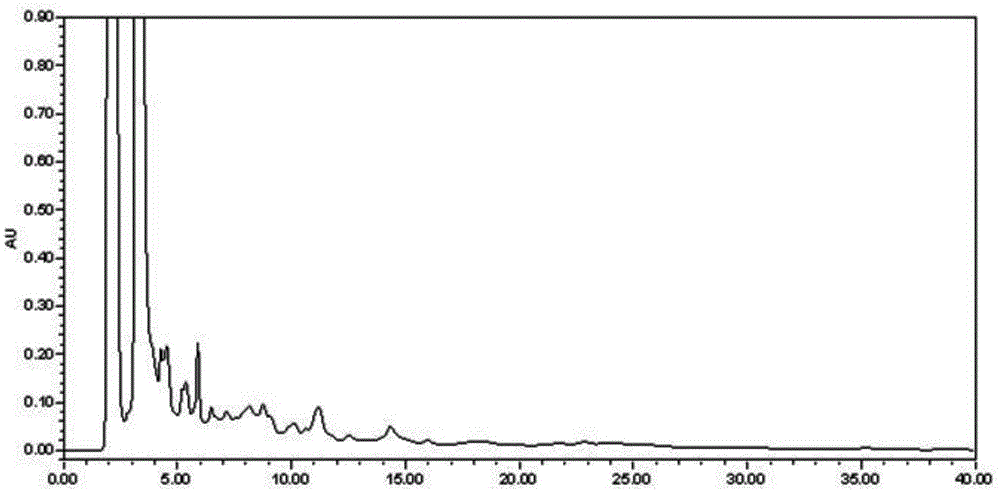
[0071] Example 3 Determination of the content of Caulis spatholobi in Kangfuling Capsules: According to Appendix VID of the 2010 edition of the Chinese Pharmacopoeia, determination:
[0072] Chromatographic conditions and system adaptability test: octadecylsilane bonded silica gel is used as a filler, acetonitrile-water=40:80 is used as the mobile phase, and the detection wavelength is 260nm;
[0073] Preparation of reference substance solution: take formononetin reference substance, dissolve it in 80% methanol, and make a solution containing 0.03mg formononetin reference substance per 1 mL as a reference substance solution;
[0074] Preparation of the test solution: Take 3g of the contents under the difference in the amount of this preparation, add 40 mL of 80% methanol, and extract for 70 minutes under reflux in a water bath, filter, evaporate the filtrate, and add 10 mL of 80% methanol to dissolve the residue. Once, take the continued filtrate as the test solution;
[0075] Determinat
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap