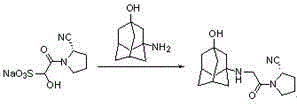
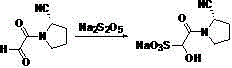Synthesis method of vildagliptin
A synthesis method and technology of a condensing agent, applied in the direction of organic chemistry, etc., can solve the problems of low purity index of the final product, low condensation reaction yield, difficult removal of by-products, etc., and achieve easy industrial production, lower production costs, and post-processing. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
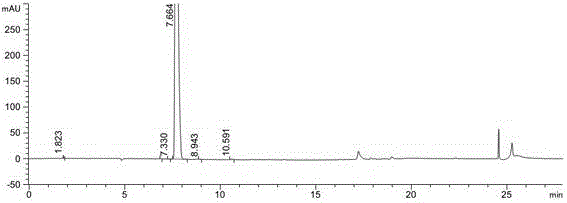
[0029] (1) Preparation of (S)-1-(2-oxoacetyl)pyrrolidine-2-carbonitrile
[0030] Add 16.2g (0.22mol) glyoxylic acid, 47.9g (0.25mol) EDCI, 33.7g (0.25mol) HOBT, 52.5g (0.52mol) triethylamine and 240 mL dichloromethane to the reaction flask, cool to 0 ℃~5℃, after stirring for 1.5h, add 20.0g (0.21mol) (S)-pyrrolidine-2-carbonitrile in batches at 0℃~5℃, then remove the ice bath, carry out at 20~25℃ The reaction was stirred for 16 hours, monitored by HPLC, and the conversion of the starting material (S)-pyrrolidine-2-carbonitrile was complete. The post-processing method is as follows: add 100 mL of water to the reaction solution, extract with dichloromethane (100 mL×2), separate the layers, wash the organic phase with 100 mL of saturated brine, dry with anhydrous sodium sulfate, and concentrate under reduced pressure to remove the solvent to obtain 28.5 g of a light yellow oily liquid. , the yield is 89%.
[0031] (2) Preparation of sodium 2-((S)-2-cyanopyrrolidin-1-yl)-1-hydroxy-
Embodiment 2
[0038] (1) Preparation of (S)-1-(2-oxoacetyl)pyrrolidine-2-carbonitrile
[0039] Add 15.5g (0.21mol) glyoxylic acid, 44.1g (0.23mol) EDCI, 28.4g (0.21mol) HOBT, 42.5g (0.42mol) triethylamine and 240 mL dichloromethane to the reaction flask, cool to 0 ℃~5℃, after stirring for 1h, add 20.0g (0.21mol) (S)-pyrrolidine-2-carbonitrile in batches at 0℃~5℃, then remove the ice bath, and carry out the reaction at 20~25℃ , stirred for 16 hours, monitored by HPLC, the conversion of the raw material (S)-pyrrolidine-2-carbonitrile was complete. The post-processing method is as follows: add 100 mL of water to the reaction solution, extract with dichloromethane (100 mL×2), separate the layers, wash the organic phase with 100 mL of saturated brine, dry over anhydrous sodium sulfate, and concentrate under reduced pressure to remove the solvent to obtain 27.9 g of a light yellow oily liquid. , the yield is 87%.
[0040] (2) Preparation of sodium 2-((S)-2-cyanopyrrolidin-1-yl)-1-hydroxy-2-oxoetha
Embodiment 3
[0045] (1) Preparation of (S)-1-(2-oxoacetyl)pyrrolidine-2-carbonitrile
[0046] Add 17.0g (0.23mol) glyoxylic acid, 47.9g (0.25mol) EDCI, 31.1g (0.23mol) HOBT, 52.5g (0.52mol) triethylamine and 240 mL dichloromethane to the reaction flask, cool to 0 ℃~5℃, after stirring for 2.5h, add 20.0g (0.21mol) (S)-pyrrolidine-2-carbonitrile in batches at 0℃~5℃, then remove the ice bath, carry out at 20~25℃ The reaction was stirred for 14 hours, monitored by HPLC, and the conversion of the starting material (S)-pyrrolidine-2-carbonitrile was complete. The post-processing method is as follows: adding 100 mL of water to the reaction solution, extracting with dichloromethane (100 mL×2) for separation, washing the organic phase with 100 mL saturated brine, drying over anhydrous sodium sulfate, and concentrating under reduced pressure to remove the solvent to obtain 27.1 g of a light yellow oily liquid. Yield 85%.
[0047] (2) Preparation of sodium 2-((S)-2-cyanopyrrolidin-1-yl)-1-hydroxy-2-ox
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap