Synthesis process of doxofylline
A technology of doxofylline and synthesis technology, applied in the field of synthesis technology of doxofylline, can solve the problems of difficulty in purification, many solvents, and high impurity content, and achieves improved reaction yield, fewer side reactions, and reduced impurity content. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
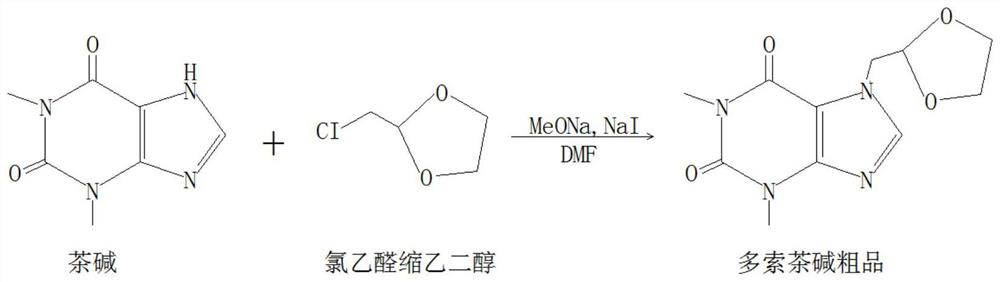
[0042] This embodiment provides a synthesis process of doxofylline, adding theophylline, halogenated acetaldehyde ethylene acetal, acid-binding agent, catalyst in the organic solvent, theophylline and halogenated acetaldehyde ethylene acetal Under the action of an acid-binding agent and a catalyst, a substitution reaction occurs in an organic solvent to generate a crude product of doxofylline. As an alternative, the acid-binding agent is an organic strong base. Preferably in this embodiment, the organic solvent is N,N-dimethylformamide (DMF), the acid-binding agent is sodium / potassium alkoxide, and the catalyst is sodium iodide. Further preferably, the acid-binding agent is sodium methoxide. As an alternative, the halogen atoms in the haloacetaldehyde ethylene acetal may be fluorine, chlorine, bromine, or iodine, and the haloacetaldehyde ethylene acetal is added at least twice. Preferably in this embodiment, in order to reduce product toxicity and reduce environmental pollut...
Embodiment 2
[0055] The present embodiment provides a kind of synthetic technique of doxofylline, comprising the following steps:
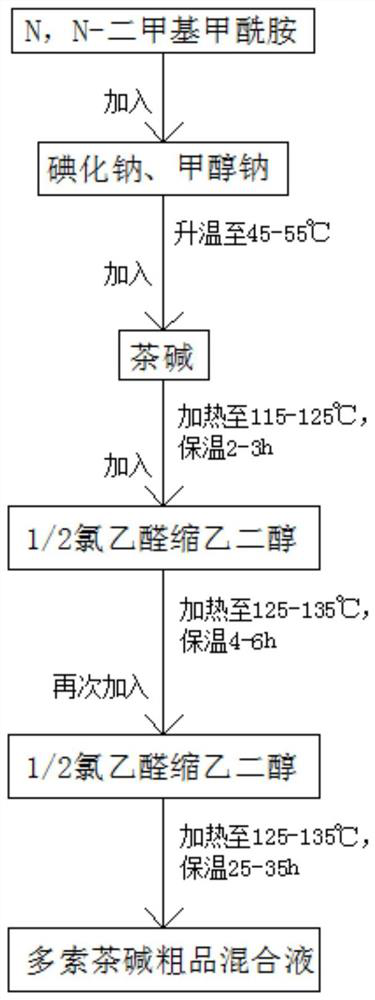
[0056] (1) Preparation of doxofylline crude product mixed solution
[0057] Synthetic route such as figure 2 As shown, add N,N-dimethylformamide into the reaction tank under vacuum, add sodium iodide and sodium methoxide under stirring, raise the temperature to 50°C, then slowly add theophylline, stir evenly, then raise the temperature to 120°C for 2.5 hours. Preferably in this embodiment, the ratio of theophylline to N,N-dimethylformamide, sodium methoxide and sodium iodide is 1:3.42:0.45:0.0067.
[0058] Then add chloroacetaldehyde ethylene acetal twice, preferably in this embodiment, the addition ratio of theophylline and chloroacetaldehyde ethylene acetal is 1:1.02. First add 1 / 2 chloroacetaldehyde ethylene acetal into the reaction tank, heat to 130°C for 5 hours, then add the remaining 1 / 2 chloroacetaldehyde ethylene glycol and continue to keep warm at...
Embodiment 3
[0065] The present embodiment provides a kind of synthetic technique of doxofylline, comprising the following steps:
[0066] (1) Preparation of doxofylline crude product mixed solution
[0067] Synthetic route such as figure 2 As shown, add N,N-dimethylformamide into the reaction tank under vacuum, add sodium iodide and sodium methoxide under stirring, raise the temperature to 55°C, then slowly add theophylline, stir evenly and then raise the temperature to 125°C for 2 hours. Preferably in this embodiment, the ratio of theophylline to N,N-dimethylformamide, sodium methoxide and sodium iodide is 1:4:0.6:0.007.
[0068] Then add chloroacetaldehyde ethylene acetal twice, preferably in this embodiment, the addition ratio of theophylline and chloroacetaldehyde ethylene acetal is 1:1.05. First add 1 / 2 chloroacetaldehyde ethylene acetal into the reaction tank, heat to 135°C for 6 hours, then add the remaining 1 / 2 chloroacetaldehyde ethylene glycol and continue to keep warm at 135...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap