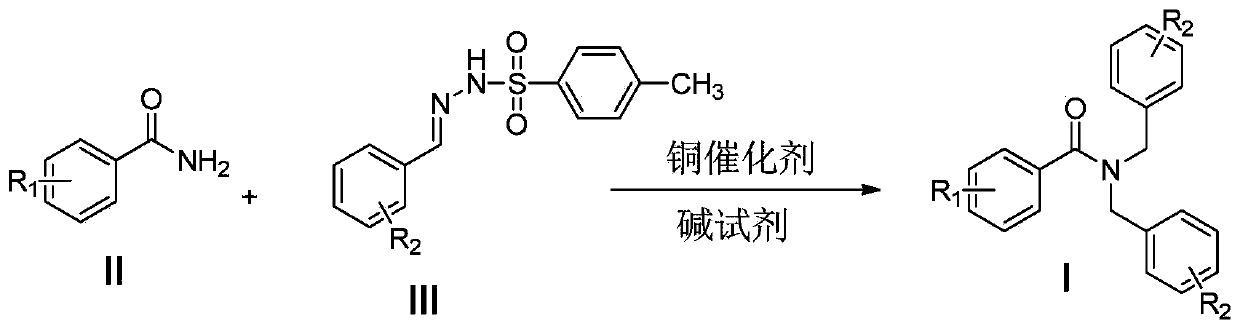
Preparation method of tertiary amide compound
A technology for tertiary amides and compounds, which is applied in the field of preparation of tertiary amide compounds, can solve problems such as expensive, achieve the effects of reducing production costs and avoiding the use of high temperatures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: the preparation of a kind of tertiary amide target compound (R 1 , R 2 =H)
[0031] Under nitrogen atmosphere, 61mg (0.5mmol) of benzamide, 616.5mg (2.25mmol) of p-toluenesulfonylhydrazone, 288mg (3.0mmol) of sodium tert-butoxide, 9.55mg (0.05mmol) of CuI, tetrabutyl iodide 18.5mg (0.05mmol) of ammonium was added to the reactor, 2.5mL of tetrahydrofuran was added, and the reaction was carried out at 100°C for 6 hours under a nitrogen atmosphere, the reaction was quenched with saturated brine, extracted with dichloromethane, dried, separated on silica gel, evaporated The methylene chloride was removed to obtain 123 mg of the product N,N-dibenzylbenzamide, and the yield was 82%.
[0032]
[0033] The yield was 82%; (column chromatography analysis, eluent petroleum ether: ethyl acetate, v / v=10 / 1). 1 H NMR (300MHz, CDCl 3 )δ(ppm)7.95-7.90(m,2H,ArH),7.62-7.54(m,3H,ArH),7.36-7.29(m,10H,ArH),,4.91(s,4H,NCH 2 ).
Embodiment 2
[0034] Embodiment 2: the preparation of a kind of tertiary amide target compound (R 1 =CH 3 , R 2 =CH 3 )
[0035]
[0036] With reference to Example 1, benzamide is replaced by equimolar p-toluenesulfonylamide, p-toluenesulfonyl hydrazone is replaced by equimolar (p-tolyl) p-toluenesulfonyl hydrazone, and other conditions remain unchanged, obtained The corresponding tertiary amide compounds.
[0037] The yield was 80%. (Column chromatography analysis, eluent petroleum ether: ethyl acetate, v / v=10 / 1). 1 H NMR (300MHz, CDCl 3 )δ(ppm)7.86-7.82(m,2H,ArH),7.34-7.30(m,2H,ArH),7.51-7.12(m,8H,ArH),4.89(s,4H,NCH 2 ),2.41(s,3H,CH 3 ),2.17(s,6H,CH 3 ).
Embodiment 3
[0038] Embodiment 3: the preparation of a kind of tertiary amide target compound (R 1 = Cl, R 2 =Br)
[0039]
[0040] With reference to Example 1, benzamide is replaced by equimolar p-chlorobenzamide, p-toluenesulfonylhydrazone is replaced by equimolar (p-bromophenyl) p-toluenesulfonylhydrazone, and other conditions remain unchanged, obtained The corresponding tertiary amide compounds.
[0041] The yield was 78% (column chromatography analysis, eluent petroleum ether: ethyl acetate, v / v=10 / 1). 1 H NMR (300MHz, CDCl 3)δ(ppm)7.90-7.85(m,4H,ArH),7.54-7.51(m,3H,ArH),7.46-7.43(m,1H,ArH),7.27-7.25(m,2H,ArH),7.19 -7.15(m,2H,ArH),4.93(s,4H,NCH 2 ).
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap