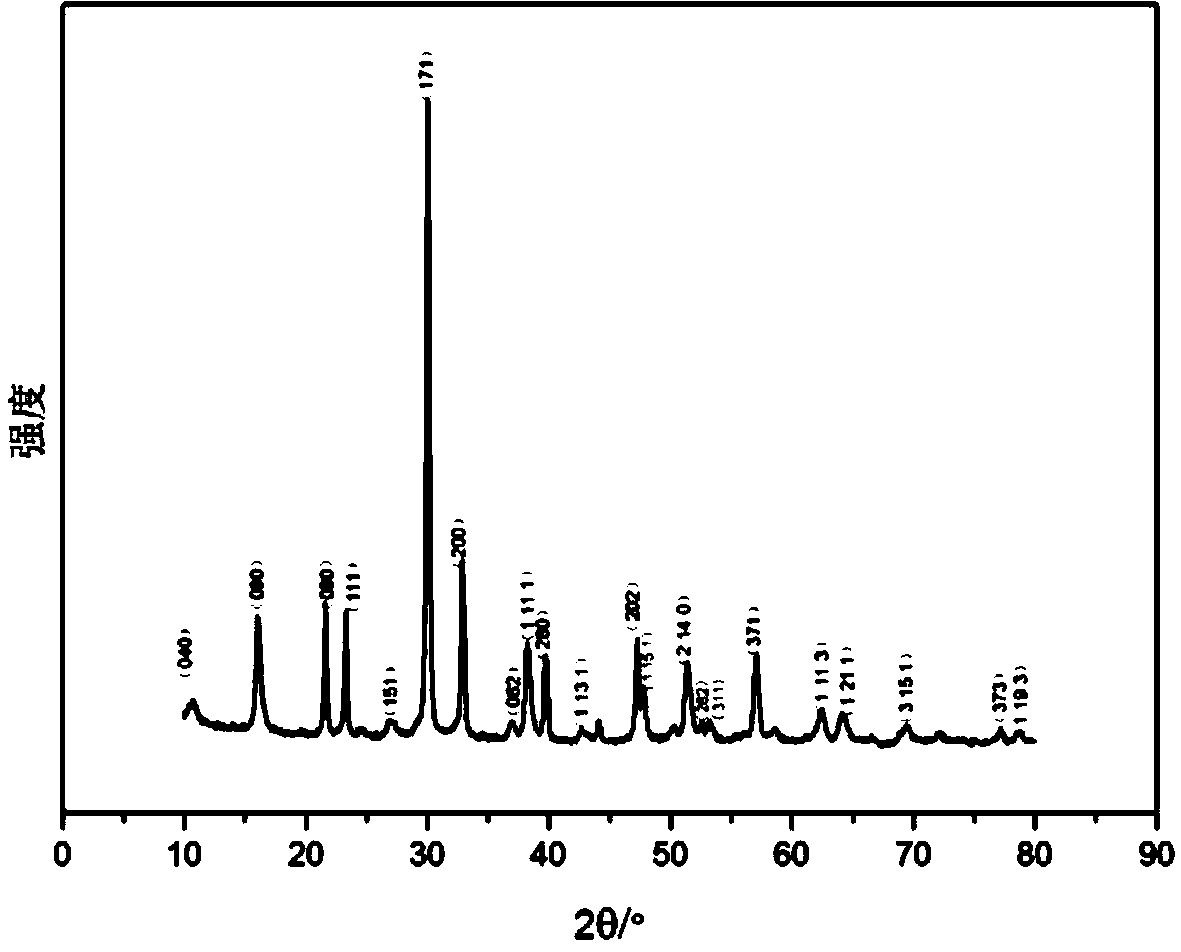
Preparation method and product of Bi4Ti3O12 micron sheets
A technology of micron flakes and concentration, applied in chemical instruments and methods, inorganic chemistry, bismuth compounds, etc., can solve the problems of difficulty in controlling the shape of bismuth titanate, serious agglomeration, etc., and achieve easy large-scale production, simple preparation process, and product purity. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1) Weigh 5.1 mmol of tetrabutyl titanate, add it dropwise into deionized water, and control the concentration of tetrabutyl titanate to 0.4 mol / L.
[0033] 2) Weigh 6.8 mmol of bismuth nitrate pentahydrate, add it into the suspension prepared in step 1), and stir thoroughly.
[0034] 3) Weigh 0.04 mol of potassium hydroxide, add to the suspension solution prepared in step 2), and stir for at least 30 minutes.
[0035] 4) Weigh 1.13 mmol polyvinyl alcohol (PVA), dissolve it in deionized water, and control the concentration of PVA to be 0.113 mol / L.
[0036] 5) In the stirring state, add the PVA solution prepared in step 4) dropwise to the suspension prepared in step 3), and disperse through sufficient stirring and ultrasonic vibration, and adjust its volume to account for the reaction with deionized water. 4 / 5 of the volume of the inner tank of the kettle, and the precursor solution is obtained at this time.
[0037] 6) Add the precursor solution prepared in step 5)
Embodiment 2
[0040] 1) Weigh 5.1 mmol of tetrabutyl titanate, add it dropwise into deionized water, and control the concentration of tetrabutyl titanate to 0.6 mol / L.
[0041] 2) Weigh 6.8 mmol of bismuth nitrate pentahydrate, add it into the suspension prepared in step 1), and stir thoroughly.
[0042] 3) Weigh 0.04 mol of potassium hydroxide, add to the suspension solution prepared in step 2), and stir for at least 30 minutes.
[0043] 4) Weigh 1.15 mmol polyvinyl alcohol (PVA), dissolve it in deionized water, and control the concentration of PVA to be 0.115 mol / L.
[0044] 5) In the stirring state, add the PVA solution prepared in step 4) dropwise to the suspension prepared in step 3), and disperse through sufficient stirring and ultrasonic vibration, and adjust its volume to account for the reaction with deionized water. 4 / 5 of the volume of the inner tank of the kettle, and the precursor solution is obtained at this time.
[0045] 6) Add the precursor solution prepared in step 5)
Embodiment 3
[0048] 1) Weigh 5.1 mmol of tetrabutyl titanate, add it dropwise into deionized water, and control the concentration of tetrabutyl titanate to 0.6 mol / L.
[0049] 2) Weigh 6.8 mmol of bismuth nitrate pentahydrate, add it into the suspension prepared in step 1), and stir thoroughly.
[0050] 3) Weigh 0.04 mol of potassium hydroxide, add to the suspension solution prepared in step 2), and stir for at least 30 minutes.
[0051] 4) Weigh 1.17 mmol polyvinyl alcohol (PVA), dissolve it in deionized water, and control the concentration of PVA to be 0.117 mol / L.
[0052] 5) In the stirring state, add the PVA solution prepared in step 4) dropwise to the suspension prepared in step 3), and disperse through sufficient stirring and ultrasonic vibration, and adjust its volume to account for the reaction with deionized water. 4 / 5 of the volume of the inner tank of the kettle, and the precursor solution is obtained at this time.
[0053] 6) Add the precursor solution prepared in step 5) i
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap