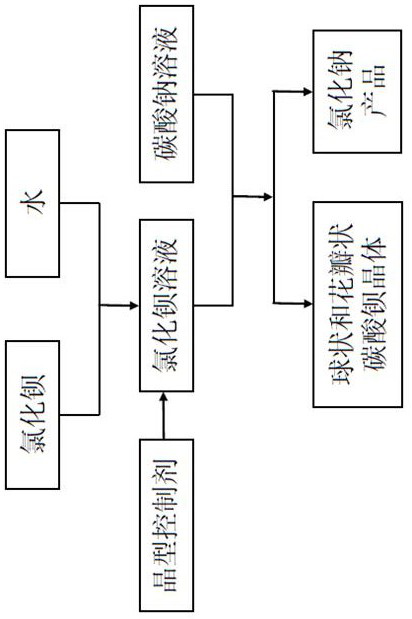
Preparation method of anhydrous barium carbonate crystals with special morphology
A barium carbonate and crystal technology is applied in the field of preparation of anhydrous barium carbonate crystals with special morphology (spherical and petal-shaped), and can solve the problem of difficult to obtain high-purity micro-nano products, difficult to control product morphology and size, complicated steps, etc. problems, to achieve the effect of increasing its own value, short response time and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Prepare a barium chloride solution with a concentration of 0.01mol / L; prepare a sodium carbonate solution with a concentration of 0.01mol / L;
[0034] Without adding a crystal form control agent, stir the magnesium chloride solution for 30 minutes to make a primary solution;
[0035] Put the primary solution in a beaker and put it into a heat-collecting constant temperature heating magnetic stirrer, heat it to 30°C, and under the stirring condition of 500r / min, add the same volume of sodium carbonate solution to the primary solution for hydrothermal reaction for 30min to obtain a white The reaction slurry of precipitation, wherein, the sodium carbonate solution add-on is the ratio metering of 1:1 by the barium ion mol ratio in carbonate ion and primary solution;
[0036] Filter the reaction slurry, take the solid phase, wash 5-7 times with deionized water, and filter until no chloride ions can be detected by using silver nitrate in the lotion to obtain the filtrate an
Embodiment 2
[0039] Method is with embodiment 1, and difference is:
[0040] Add the crystal form control agent to the barium chloride solution, stir for 30min, and make a primary solution; the crystal form control agent is citric acid, and its addition is 10% of the total weight of barium chloride dihydrate;
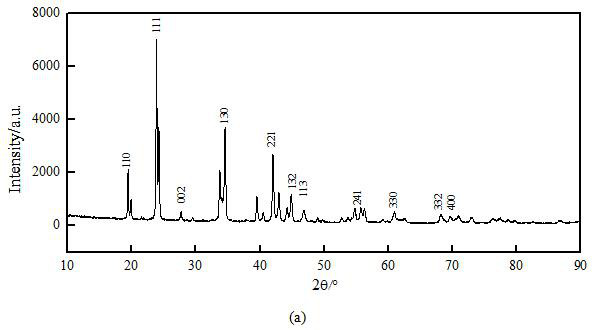
[0041] Dry the white precipitate in an electric blast drying oven at 80°C to produce rod-shaped anhydrous barium carbonate crystals. After testing, the purity of the rod-shaped anhydrous barium carbonate crystals is 98%, and the particle size is microns. SEM, XRD and FTIR photos Such as figure 2 Shown in (b), 3(b) and 4(b); The yield of rod-shaped anhydrous barium carbonate crystal is 98%.
Embodiment 3
[0043] Method is with embodiment 1, and difference is:
[0044] Add the crystal form control agent to the barium chloride solution and stir for 30 minutes to make a primary solution; the crystal form control agent is disodium ethylenediaminetetraacetic acid (EDTA-2Na), and its addition amount is 10% of the total weight of barium;
[0045] Dry the white precipitate in an electric blast drying oven at 80°C to produce rod-shaped anhydrous barium carbonate crystals. After testing, the purity of the rod-shaped anhydrous barium carbonate crystals is 98%, and the particle size is microns. SEM, XRD and FTIR photos Such as figure 2 Shown in (c), 3(c) and 4(c); The yield of rod-shaped anhydrous barium carbonate crystal is 98%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap