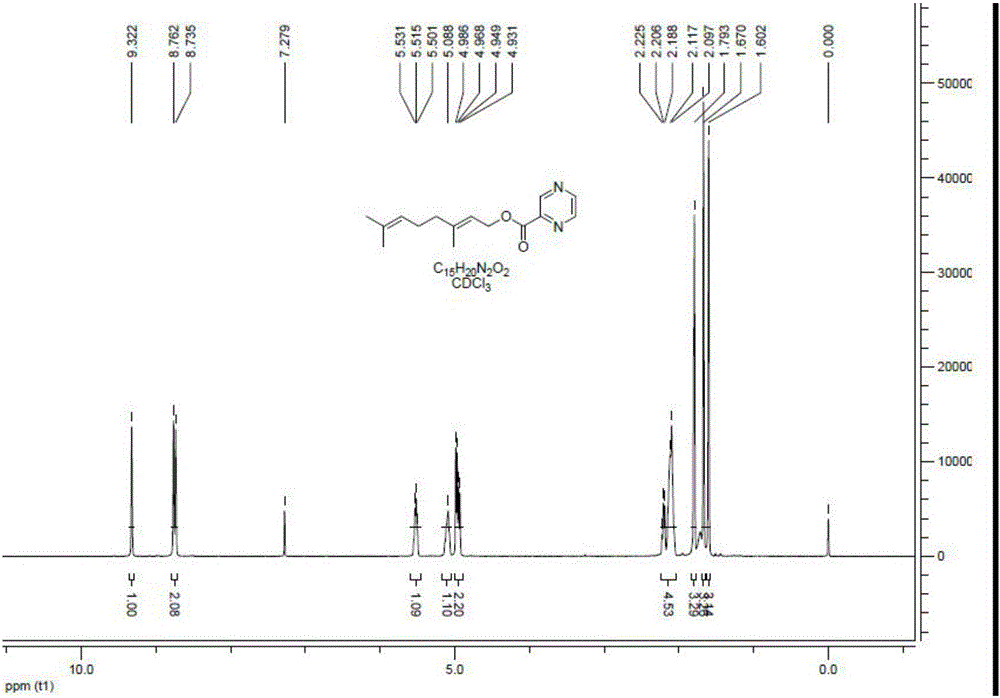
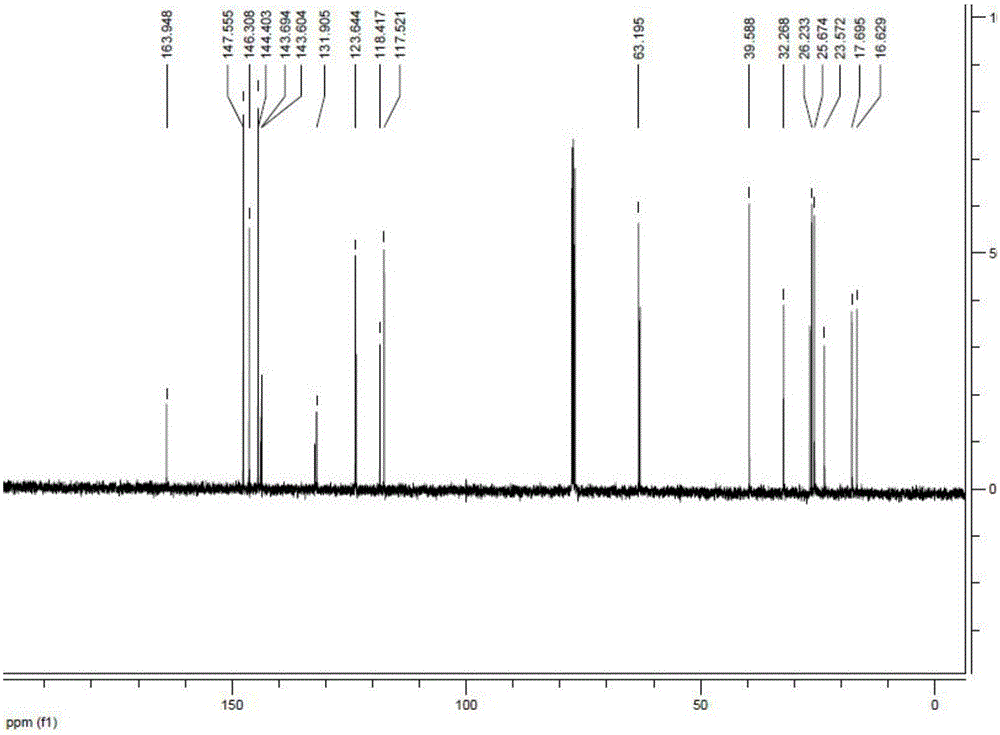
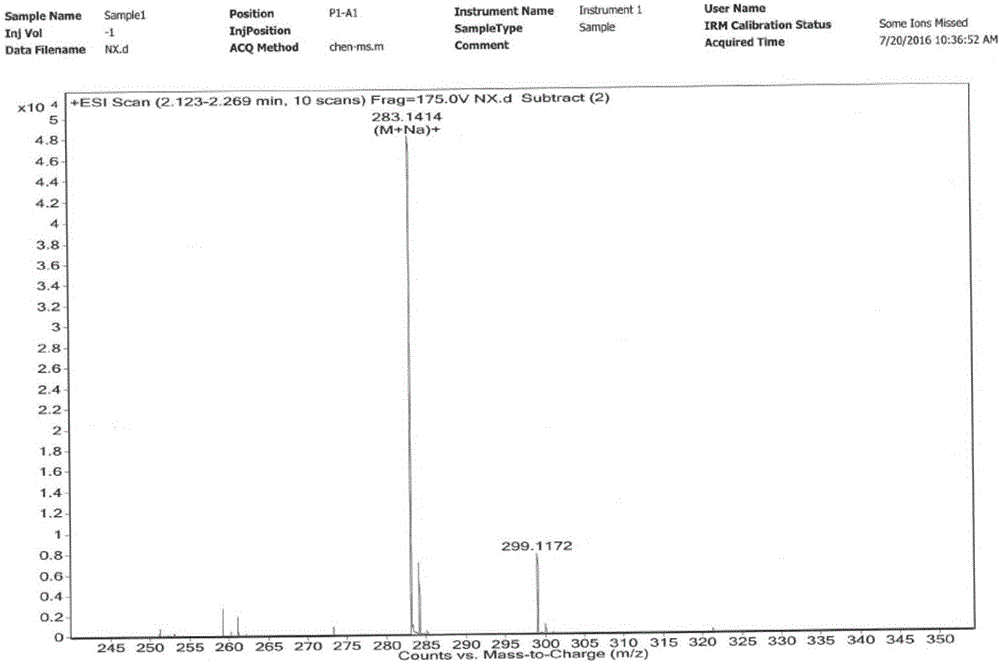
Preparation method and application of tobacco precursor-aroma monomer pyrazine-2-formate
A technology of pyrazine and formic acid, applied in the field of flavors and fragrances and tobacco processing, can solve the problems of short fragrance retention time, poor high temperature thermal stability, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The preparation method of pyrazine-2-carboxylic acid geraniol ester of tobacco flavor monomer:
[0027] Take 10mmol of geraniol and 10mmol of pyrazine-2-carboxylic acid dissolved in 100ml of dry dichloromethane, after stirring for 10min, add 4-dimethylaminopyridine (DMAP) (5mmol) and 1-ethyl-(3-dimethyl Aminopropyl) carbodiimide hydrochloride (EDCHCl) (11mmol), stirring at room temperature, TLC monitoring reaction (petroleum ether: ethyl acetate = 10:1, Rf = 0.5, ultraviolet 254nm color development), tracking reaction end. Add 50 mL of water to the organic phase to wash, separate the layers, then wash with 50 mL of saturated sodium chloride solution, separate the layers, dry the organic phase with anhydrous Na2SO4, and concentrate to obtain a crude product. Dissolve the crude product in 50 mL of dichloromethane, add 4 g of 100-200 mesh silica gel, spin dry the sample under reduced pressure, and use 80 g of 100-200 mesh silica gel chromatography to slowly elute with petrol
Embodiment 2
[0029] The preparation method of pyrazine-2-carboxylic acid geraniol ester of tobacco flavor monomer:
[0030] Take 10mmol of geraniol and 10.5mmol of pyrazine-2-carboxylic acid dissolved in 100ml of dry dichloromethane, after stirring for 10min, add 4-dimethylaminopyridine (DMAP) (4mmol) and 1-ethyl-(3-dichloromethane Methylaminopropyl) carbodiimide hydrochloride (EDCHCl) (11 mmol), stirred at room temperature, TLC monitoring reaction (petroleum ether: ethyl acetate = 10:1, Rf = 0.5, ultraviolet 254nm color development), Track reaction endpoints. Add 50 mL of water to the organic phase to wash, separate the layers, then wash with 50 mL of saturated sodium chloride solution, separate the layers, dry the organic phase with anhydrous Na2SO4, and concentrate to obtain a crude product. Dissolve the crude product in 50 mL of dichloromethane, add 4 g of 100-200 mesh silica gel, spin dry the sample under reduced pressure, and use 80 g of 100-200 mesh silica gel chromatography to slowly
Embodiment 3
[0032] The preparation method of tobacco fragrance monomer pyrazine-2-formic acid-β-ionyl ester:
[0033] In a three-necked flask, add weighed 20mmol β-ionol and 20mmol pyrazine-2-carboxylic acid dissolved in 200ml dry methylene chloride, after stirring for 10min, add 4-dimethylaminopyridine (DMAP) (9mmol) and 1- Ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC·HCl) (22mmol), stirring at room temperature, TLC monitoring reaction (petroleum ether: ethyl acetate = 10:1, Rf =0.5, ultraviolet 254nm color), follow the reaction to the end point. Add 100 mL of pure water to the organic phase to wash, separate the layers, then wash with 100 mL of saturated sodium chloride solution, separate the layers, dry the organic phase with anhydrous Na2SO4, and concentrate the crude product. The crude product was dissolved in 10 mL of dichloromethane, 7 g of 100-200 mesh silica gel was added, the sample was spin-dried under reduced pressure, and the sample was separated by 100 g of 100
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap