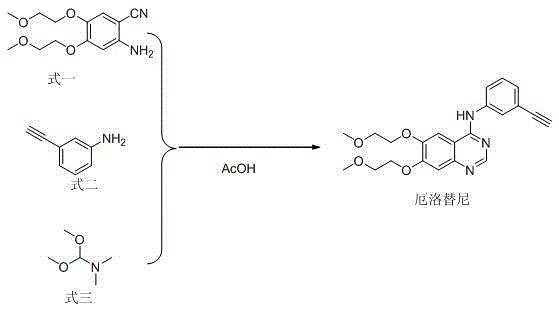
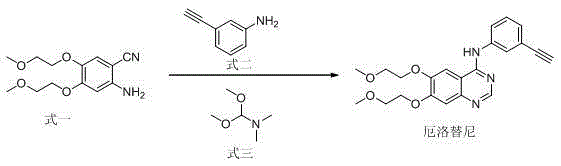
Method for preparing erlotinib
An erlotinib and compound technology, which is applied in the field of synthesis for the preparation of erlotinib, can solve problems such as unfavorable purification and quality research, unstable placement, unfavorable storage, etc., achieves simple operation, avoids long reaction steps, and achieves quality control. handy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
reference example 1
[0020] Reference example 1 Preparation of the compound of formula one (refer to the patent "WO2007138613" EXAMPLES (d))
[0021] Add 10g of 4,5-bis(2-methoxyethoxy)-2-nitro-benzonitrile to 75mL of acetic acid and 75mL of water, stir for 10min, add 7g of iron powder in batches, after the addition is complete, stir at 30°C React for 30 minutes. After the reaction was completed, adjust the pH to 7 with alkaline water, extract with ethyl acetate, dry the ethyl acetate layer over anhydrous sodium sulfate, concentrate, and recrystallize from methanol to obtain 6 g of the compound of formula 1.
Embodiment 1
[0022] Embodiment 1 Preparation of Erlotinib
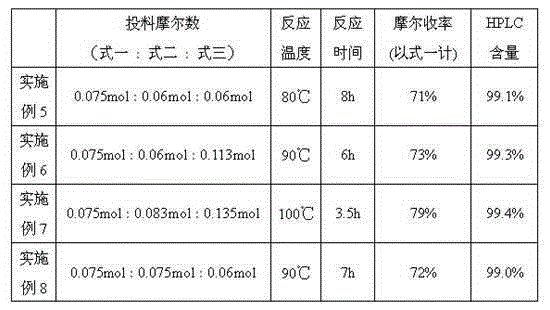
[0023] Add formula 1 (20.0g, 0.075mol), formula 2 (8.8g, 0.075mol), formula 3 (10.7g, 0.09mol), and 200mL of acetic acid in a 250mL three-neck flask. After the addition, the temperature was raised to 100°C for 3 hours. After the reaction was completed, the reaction solution was concentrated to about 100mL, poured into 200mL of ice water, then adjusted to pH 7~8 with NaOH aqueous solution, and the solid was precipitated, filtered with suction, and the filter cake was recrystallized from 100mL of ethyl acetate to obtain 23.6g of erlotinib, mole Yield 80%, HPLC content 99.2%.
Embodiment 2
[0024] Embodiment 2 Preparation of Erlotinib
[0025] Add formula 1 (20.0g, 0.075mol), formula 2 (9.7g, 0.0825mol), formula 3 (17.9g, 0.15mol), and 200mL of acetic acid into a 250mL three-neck flask in sequence. After the addition, the temperature was raised to 110°C for 2 hours. After the reaction was completed, the reaction solution was concentrated to about 100mL, poured into 200mL of ice water, then adjusted to pH 7~8 with NaOH aqueous solution, and the solid was precipitated, filtered by suction, and the filter cake was recrystallized from 100mL of ethyl acetate to obtain 24.5g of erlotinib, mol The yield is 83% (based on formula 1), and the HPLC content is 99.5%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap