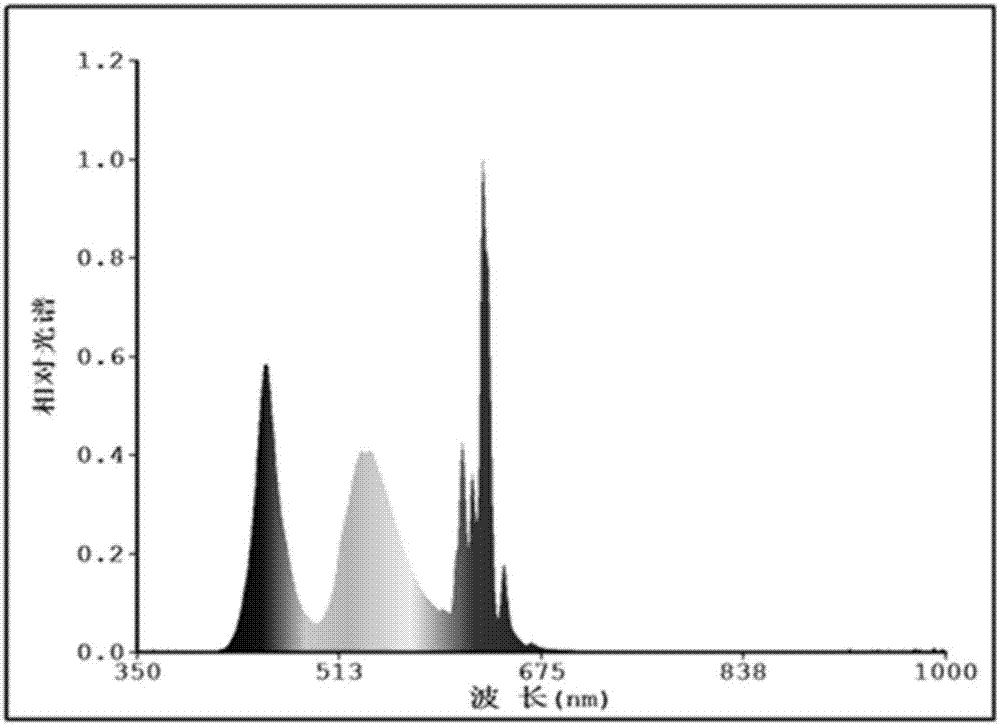
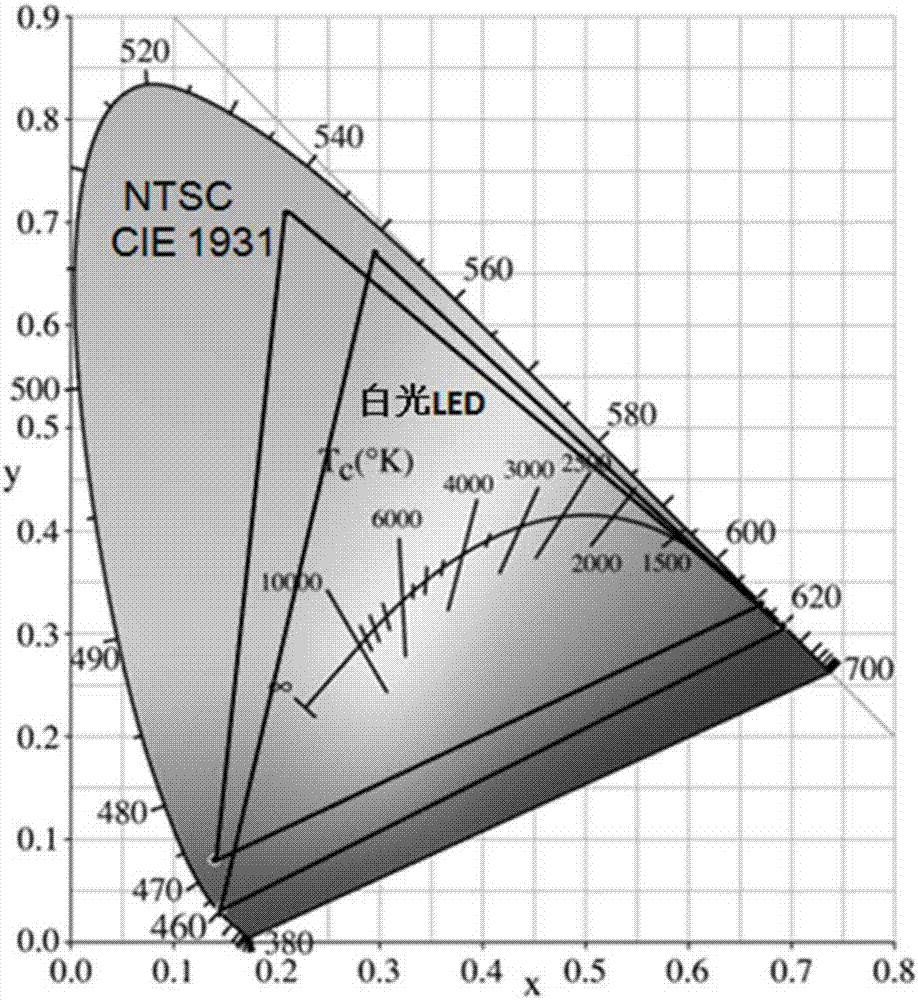
Mn<4+> doped fluoroaluminate red fluorescent powder as well as preparation method and application thereof
A red phosphor, fluoroaluminate technology, applied in chemical instruments and methods, luminescent materials, electrical components, etc., can solve problems such as instability, and achieve the effects of phase purity, wide color gamut, and high luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] a kind of Mn 4+ The preparation method of doped fluoroaluminate red fluorescent powder is characterized in that the steps are as follows: add hydrofluoric acid and water into a polytetrafluoroethylene container, and then add K 2 MnF 6 , stir to make it dissolve and then add KF; after KF dissolves, add K 3 AlF 6 Continue to stir until K 3 AlF 6 Continue stirring for 30 minutes after complete dissolution; stop stirring for 6 hours, filter with suction, take the filter cake and dry it under vacuum at 70°C to obtain the Mn 4+ Doped fluoroaluminate red phosphor. K 3 AlF 6 Can not exist stably in aqueous solution, partly decomposed into K 2 AlF 5 ·H 2 O, add KF·2H to the reaction system 2 O helps suppress K 3 AlF 6 decomposition to synthesize pure phase K 3 AlF 6 .
[0025] KF and K 3 AlF 6 The molar ratio is 0.4:1, K 2 MnF 6 with K 3 AlF 6 The molar ratio is 0.02:1.
[0026] The mass concentration of described hydrofluoric acid is 10%, and the mass concen
Embodiment 2
[0028] a kind of Mn 4+ The preparation method of doped fluoroaluminate red fluorescent powder is characterized in that the steps are as follows: add hydrofluoric acid and water into a polytetrafluoroethylene container, and then add K 2 MnF 6 , stir to make it dissolve and then add KF; after KF dissolves, add K 3 AlF 6 Continue to stir until K 3 AlF 6 Continue stirring for 30 minutes after complete dissolution; stop stirring for 24 hours, filter with suction, take the filter cake and dry it under vacuum at 70°C to obtain the Mn 4+ Doped fluoroaluminate red phosphor. K 3 AlF 6 Can not exist stably in aqueous solution, partly decomposed into K 2 AlF 5 ·H 2 O, add KF·2H to the reaction system 2 O helps suppress K 3 AlF 6 decomposition to synthesize pure phase K 3 AlF 6 .
[0029] KF and K 3 AlF 6 The molar ratio is 6:1, K 2 MnF 6 with K 3 AlF 6 The molar ratio is 0.05:1.
[0030] The mass concentration of the hydrofluoric acid is 10% to 40%, the mass concentrat
Embodiment 3
[0032] a kind of Mn 4+ The preparation method of doped fluoroaluminate red fluorescent powder is characterized in that the steps are as follows: add hydrofluoric acid and water into a polytetrafluoroethylene container, and then add K 2 MnF 6 , stir to make it dissolve and then add KF; after KF dissolves, add K 3 AlF 6 Continue to stir until K 3 AlF 6 Continue stirring for 30 minutes after complete dissolution; stop stirring for 18 hours, filter with suction, take the filter cake and dry it under vacuum at 70°C to obtain the Mn 4+ Doped fluoroaluminate red phosphor. K 3 AlF 6 Can not exist stably in aqueous solution, partly decomposed into K 2 AlF 5 ·H 2 O, add KF·2H to the reaction system 2 O helps suppress K 3 AlF 6 decomposition to synthesize pure phase K 3 AlF 6 .
[0033] KF and K 3 AlF 6 The molar ratio is 2:1, K 2 MnF 6 with K 3 AlF 6 The molar ratio is 0.03:1. The mass concentration of hydrofluoric acid is 20%, and the mass concentration of solute in
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap