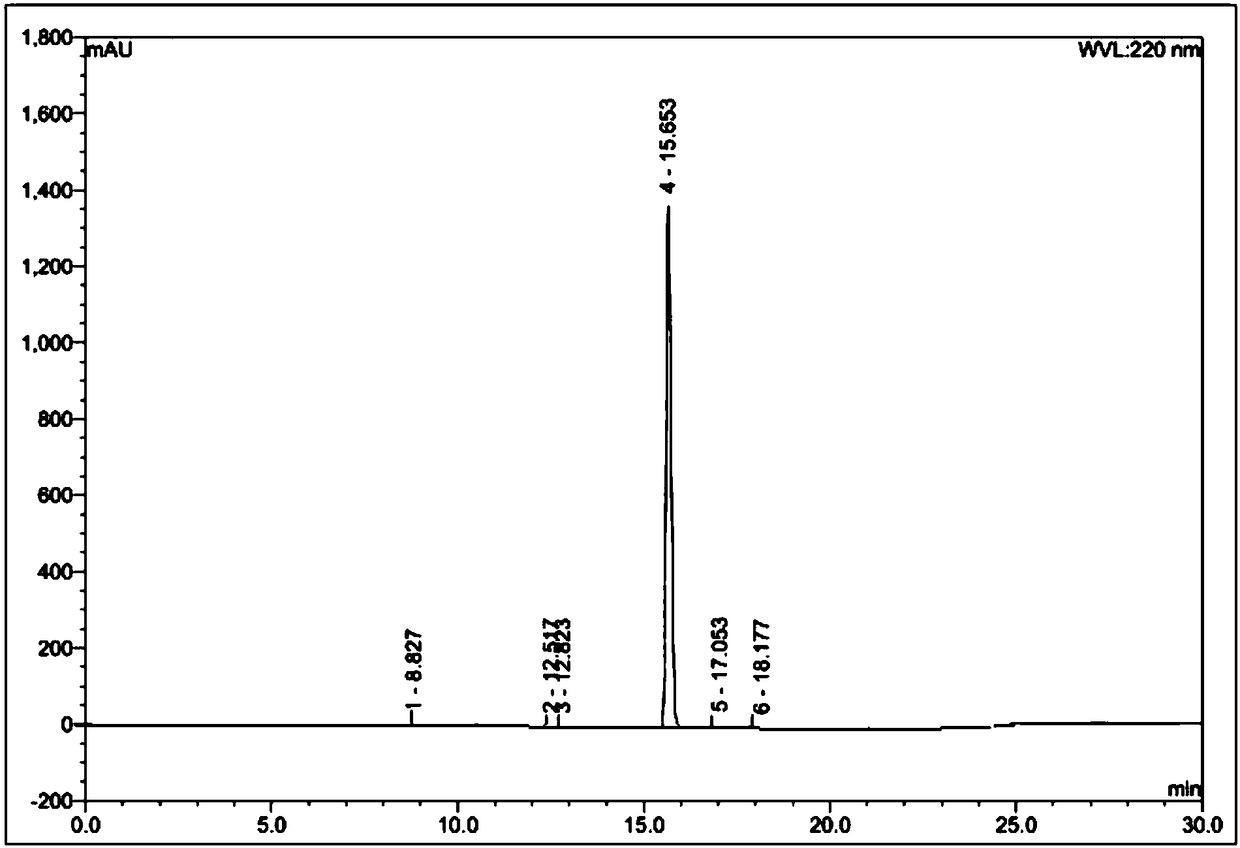
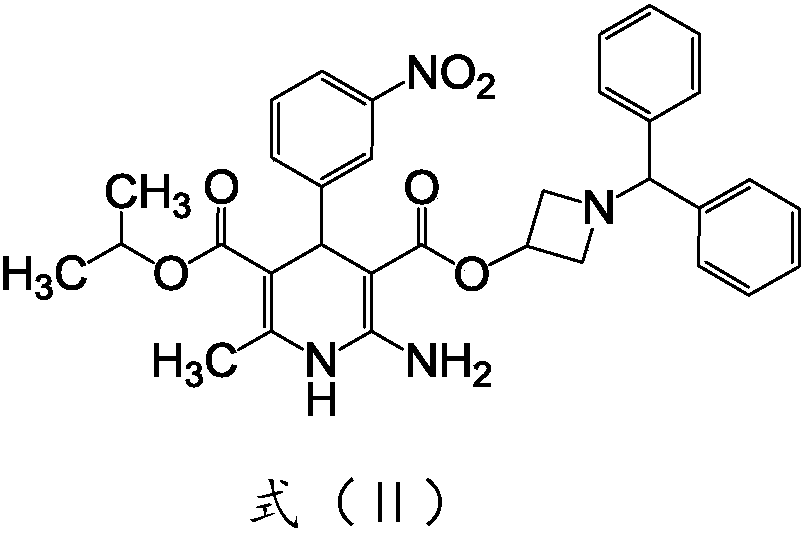
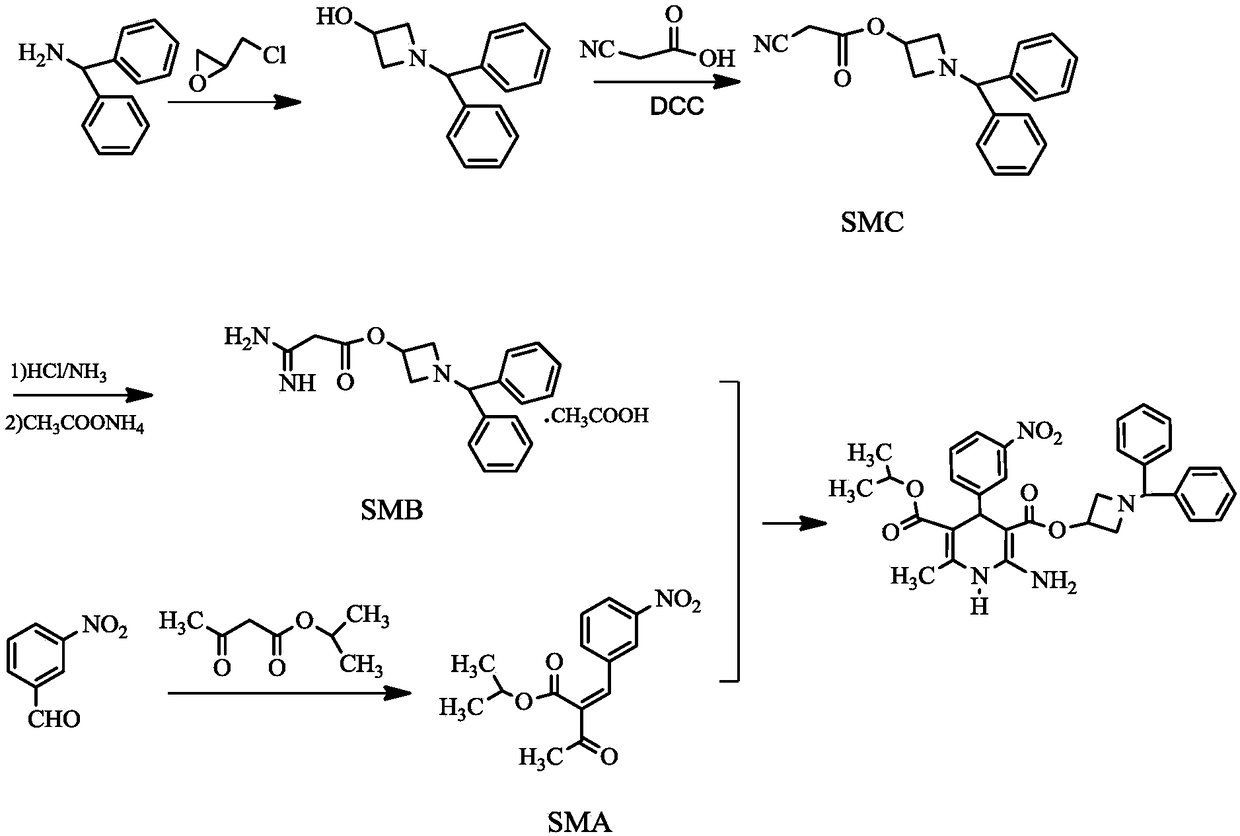
Azelnidipine impurity and preparation method thereof
A technology for azeldipine and impurities, which is applied in the field of azeldipine impurities and its preparation, can solve the problems of many side reactions, difficult separation of target products, low yield and the like, and achieves high product yield and purity, easy separation and reaction. mild effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The preparation method of embodiment 1 Azedipine impurity
[0034] Dissolve 3.83g of azedipine intermediate SMB in 50ml of water, add dropwise saturated potassium carbonate aqueous solution to adjust the pH to 9, extract with 50ml of toluene, and dry the toluene with anhydrous magnesium sulfate and molecular sieves after liquid separation;
[0035] Add the toluene solution into a 150ml reaction flask, add 1.54g of ammonium acetate, add dropwise 10ml of 2M triethylaluminum toluene solution under ice cooling, raise the temperature to 40°C after the addition, and react for 16 hours. After the reaction was completed, the temperature was lowered, filtered, the filter cake was washed with a little toluene, and dried at 40°C to obtain 1.54 g of malonamide acetate, with a yield of 95.6%;
[0036] Add 1.54g of malonamide acetate and 2.64g of azendipine intermediate SMA into a 100ml reaction flask, add 50ml of ethanol and 1.03g of sodium ethoxide, and heat up to 45°C for 7 hours. A
Embodiment 2
[0039] The preparation method of embodiment 2 Azedipine impurity
[0040] Dissolve 3.83g of azedipine intermediate SMB in 50ml of water, add dropwise saturated aqueous sodium bicarbonate solution to adjust the pH to 14, extract with 50ml of toluene, and dry the toluene with anhydrous calcium sulfate once after liquid separation;
[0041] Add the toluene solution into a 150ml reaction flask, add 2.14g of ammonium chloride, add dropwise 20ml of 2M trimethylaluminum toluene solution under ice bath, raise the temperature to 60°C after the addition, and react for 24 hours. After the reaction was completed, cool down, filter, wash the filter cake with a little toluene, and dry at 60°C to obtain 1.32 g of malonamide hydrochloride, with a yield of 96.5%;
[0042] Add 1.32g of malonamidine hydrochloride and 2.67g of azendipine intermediate SMA into a 100ml reaction flask, add 50ml of isopropanol and 1.03g of sodium methoxide, and heat up to 65°C for 10 hours. After the reaction was compl
Embodiment 3
[0044] The preparation method of embodiment 3 Azedipine impurity
[0045] Dissolve 3.83g of azeldipine intermediate SMB in 50ml of water, and adjust the pH to 12 by adding a saturated potassium carbonate aqueous solution dropwise. Extract with 50ml of toluene, and dry the toluene with anhydrous magnesium sulfate and molecular sieves after separation.
[0046] Add the toluene solution into a 150ml reaction flask, add 3.45g of ammonium bisulfate, add dropwise 15ml of 2M trimethylaluminum toluene solution under ice cooling, raise the temperature to 50°C after the addition, and react for 20 hours. After the reaction was completed, the temperature was lowered, filtered, the filter cake was washed with a little toluene, and dried at 50° C. to obtain 1.93 g of malonamidine bisulfate with a yield of 97.6%.
[0047] Add 1.93g of malonamidine hydrogen sulfate and 2.70g of azedipine intermediate SMA into a 100ml reaction flask, add 50ml of methanol and 1.03g of sodium methoxide, and heat u
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap