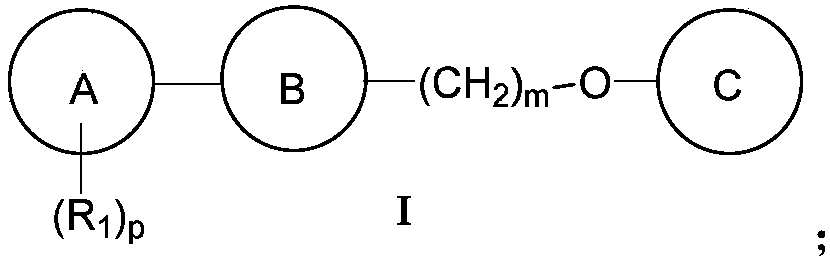
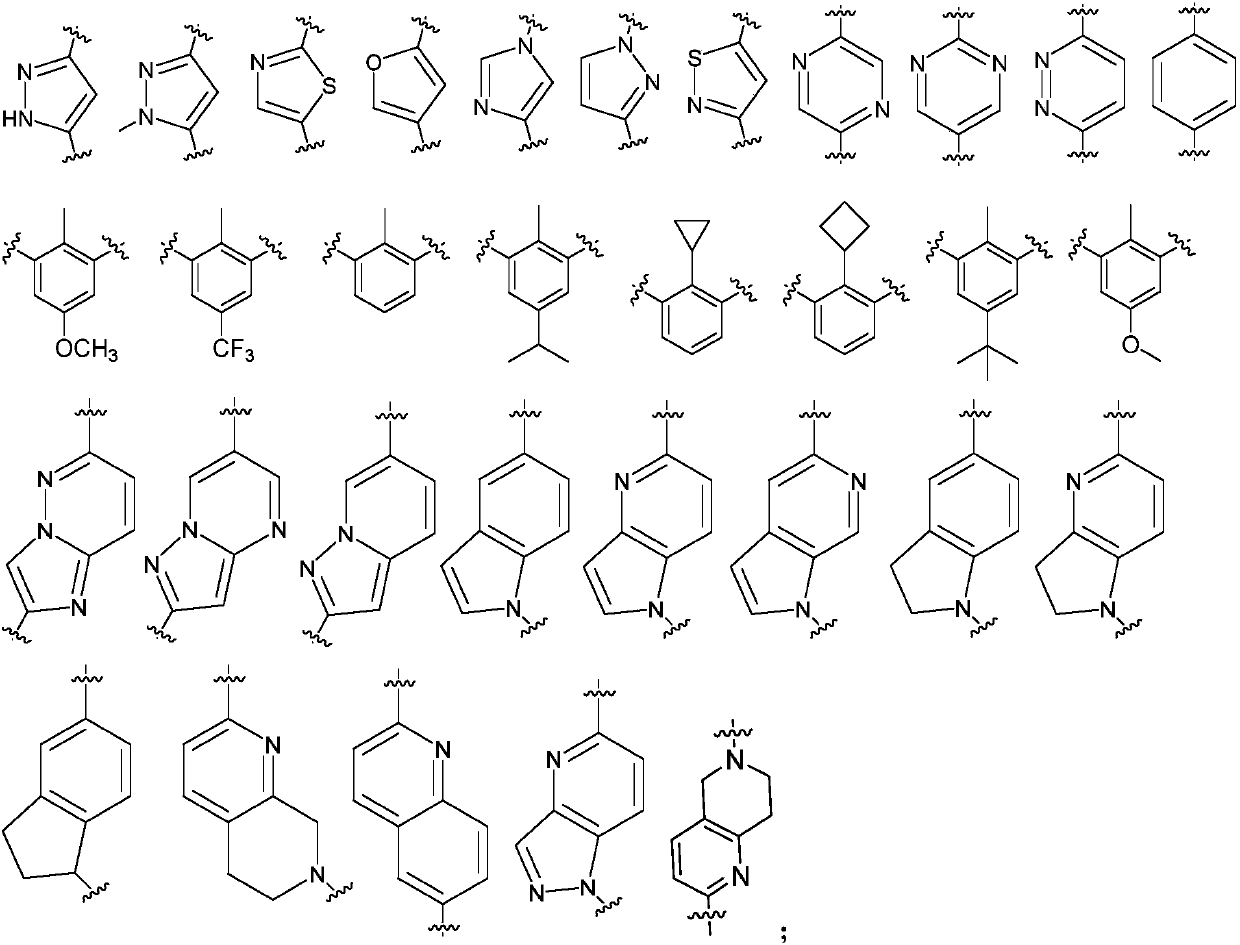
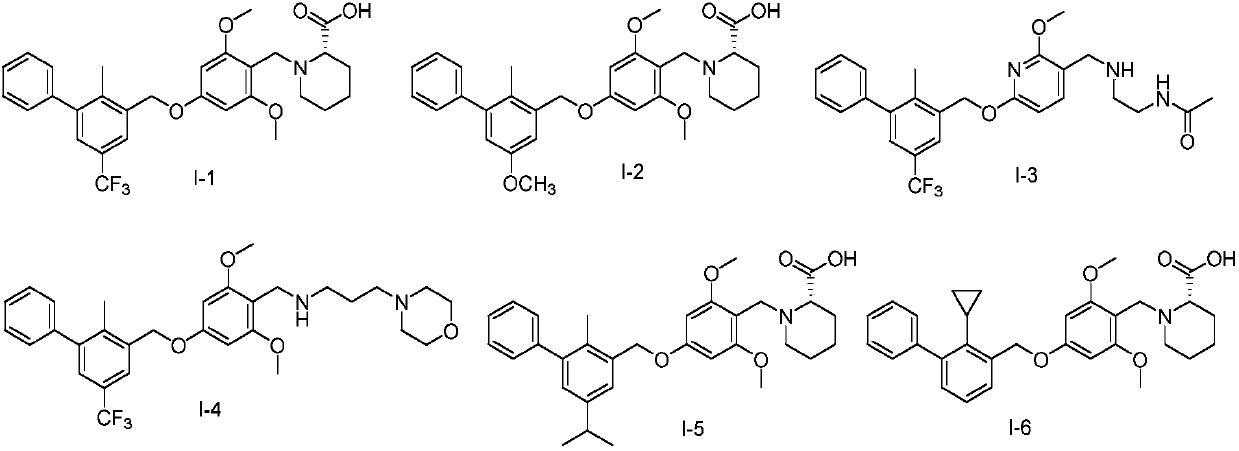
Novel anti-PD-L1 compound and applications thereof, and composition containing novel anti-PD-L1 compound
A compound and hydrate technology, applied in the field of biomedicine, can solve problems such as low bioavailability and poor therapeutic activity of solid tumors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0136] Example 1 Preparation of Compound I-1
[0137]
[0138] Compound I-1a (43 mg, 0.1 mmol), S-piperidine-2-carboxylic acid (13.6 mg, 0.105 mmol), and sodium triacetoxyborohydride (21.2 mg, 0.25 mmol) were added to 10 mL of dichloromethane solution The crude product was purified by preparative LC / MS with stirring at 80-85°C for 1 hour, and its purity was estimated to be 98% by LCMS analysis.
Embodiment 2
[0139] Example 2 Preparation of Compound I-14
[0140]
[0141] Compound I-14a (39 mg, 0.1 mmol), piperidine-2-carboxylic acid (13.6 mg, 0.105 mmol), and sodium triacetoxyborohydride (21.2 mg, 0.25 mmol) were added to 10 mL of dichloromethane solution, After stirring at 80-85°C for 45 minutes, the crude product was purified by preparative LC / MS and evaluated to be 97% pure by LCMS analysis.
Embodiment 3
[0142] Example 3 Preparation of compound I-20
[0143]
[0144] Compound I-20a (35 mg, 0.1 mmol), piperidine-2-carboxylic acid (13.6 mg, 0.105 mmol), and sodium triacetoxyborohydride (21.2 mg, 0.25 mmol) were added to 10 mL of dichloromethane solution, After stirring at 80-85°C for 50 minutes, the crude product was purified by preparative LC / MS and evaluated to be 98% pure by LCMS analysis.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap