Roxadustat intermediate impurity compound as well as preparation method and application thereof
A technology of roxadustat and compounds, applied in the field of drug synthesis, capable of solving problems such as unreported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
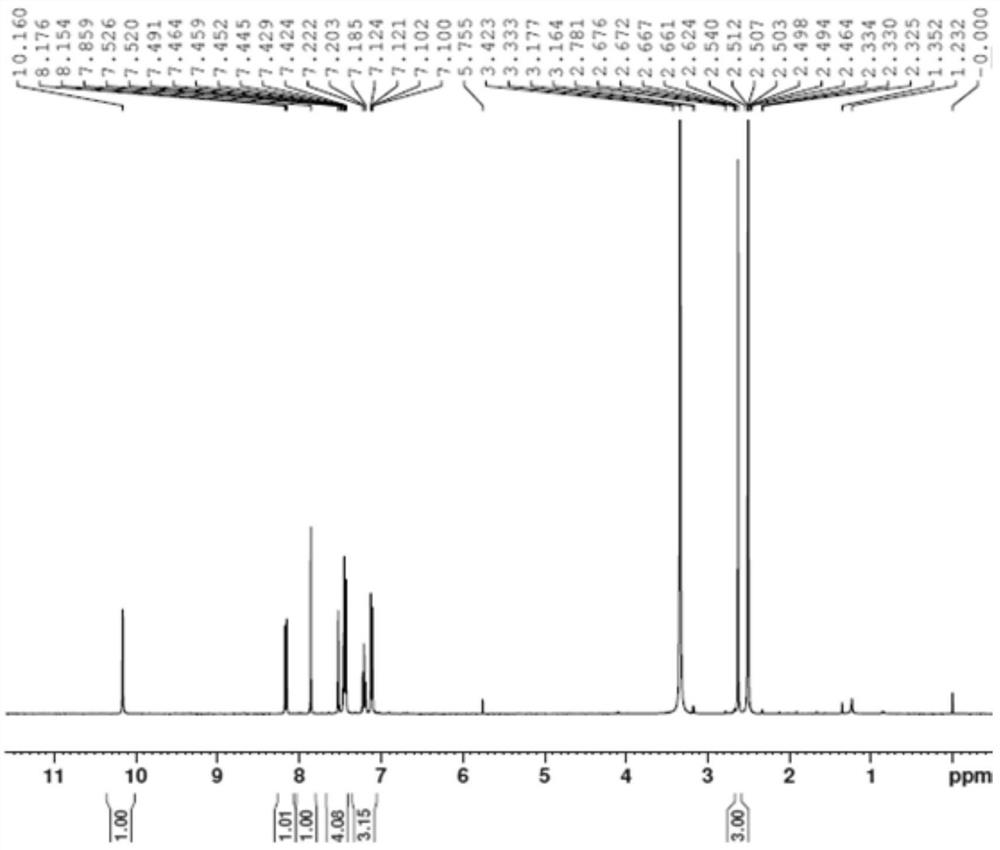
[0039] The preparation of formula I compound:
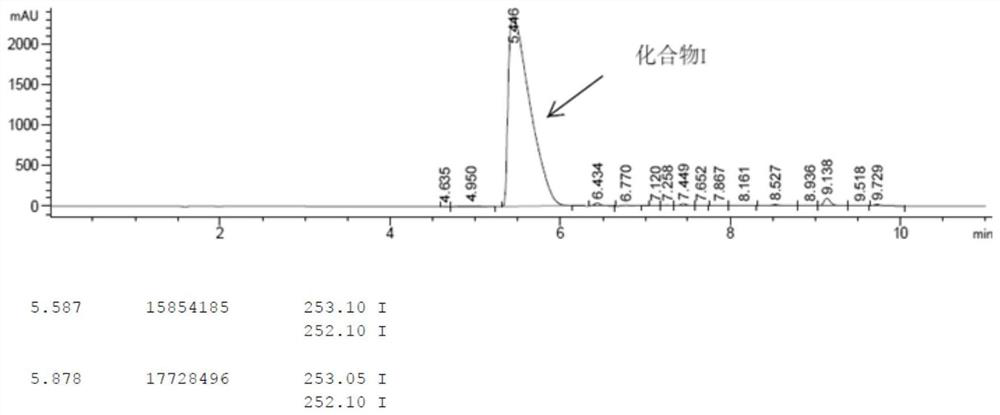
[0040] Add 10.0g (II) of methyl 1-methyl-4-hydroxy-7-phenoxyisoquinoline-3-carboxylate into a 100ml reaction flask, add 20ml of methanol, and add the previously prepared potassium hydroxide aqueous solution (8.65g of potassium hydroxide was dissolved in 20ml of water), the temperature was raised to reflux, and the temperature was kept overnight, and no raw material was detected by TLC; 5.6g of hydrochloric acid was added to adjust the pH to 7-8. The reaction solution was concentrated, 30 ml of ethyl acetate was added, 15 ml of water was added to extract and separate layers, and the ethyl acetate phase was concentrated to obtain 5.8 g of crude compound I, with a yield of 84.9% and a HPLC purity of 75.2%;
[0041] Add 5.8g of crude compound I to a 100ml three-neck flask, add 10ml of tetrahydrofuran, heat to 65°C, stir to dissolve, add dropwise 20ml of n-heptane, cool down to 0°C; filter with suction and concentrate to obtain 4.1g of p
Embodiment 2
[0043] The preparation of formula I compound:
[0044] Add 10.0g (II) of methyl 1-methyl-4-hydroxy-7-phenoxyisoquinoline-3-carboxylate into a 100ml reaction flask, add 20ml of acetonitrile, and add the previously prepared barium hydroxide aqueous solution (15.6 g of barium hydroxide was dissolved in 25 ml of water), the temperature was raised to reflux, and the temperature was kept overnight, and no raw materials were detected by TLC; 3.3 g of hydrochloric acid was added to adjust the pH to 7-8. Concentrate the reaction solution, add 30ml of ethyl acetate, add 15ml of water to extract and separate layers, concentrate the ethyl acetate phase to obtain 4.8g of compound I crude product, yield 79.0%, HPLC purity: 82.1%;
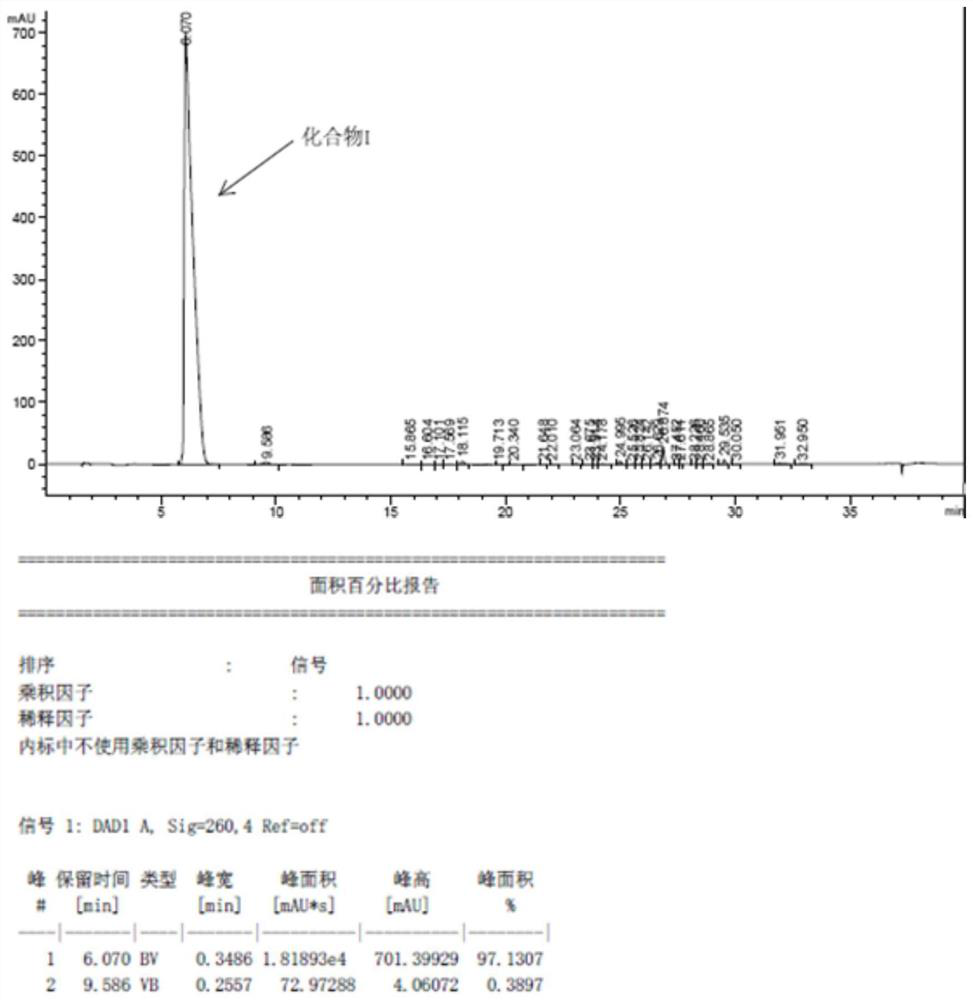
[0045] Add 4.8g of crude compound I to a 100ml three-neck flask, add 10ml of ethyl acetate, heat to 65°C, stir to dissolve, add dropwise 20ml of n-heptane, cool down to 0°C; filter with suction and concentrate to obtain the purified compound I 3.5 g, 75% recovery,
Embodiment 3
[0047] The preparation of formula I compound:
[0048] Add 10.0g (II) of methyl 1-methyl-4-hydroxy-7-phenoxyisoquinoline-3-carboxylate into a 100ml reaction flask, add 20ml of methanol, and add the previously prepared potassium hydroxide aqueous solution (8.5 g of potassium hydroxide was dissolved in 15 ml of water), the temperature was raised to reflux, and the temperature was kept overnight, and no raw material was detected by TLC; 5.6 g of hydrochloric acid was added to adjust the pH to 7-8. Concentrate the reaction solution, add 30ml of ethyl acetate, add 15ml of water to extract and separate layers, concentrate the ethyl acetate phase to obtain 4.5g of compound I crude product, yield 79.0%, HPLC purity: 76.5%;
[0049] Add 4.5g of crude compound I to a 100ml three-neck flask, add 10ml of tetrahydrofuran, heat to 65°C, stir to dissolve, add dropwise 20ml of methyl tert-butyl ether, cool down to 0°C; filter with suction and concentrate to obtain purified compound I Product 3.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap