Ester Compound and Its Use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
production example
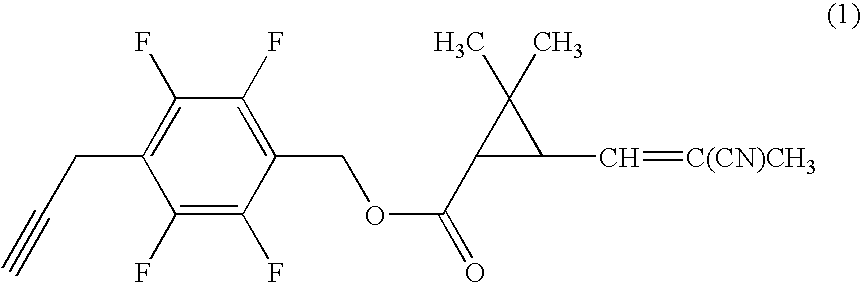
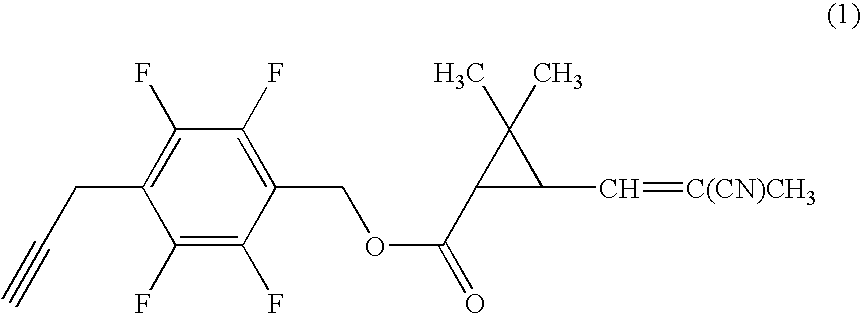
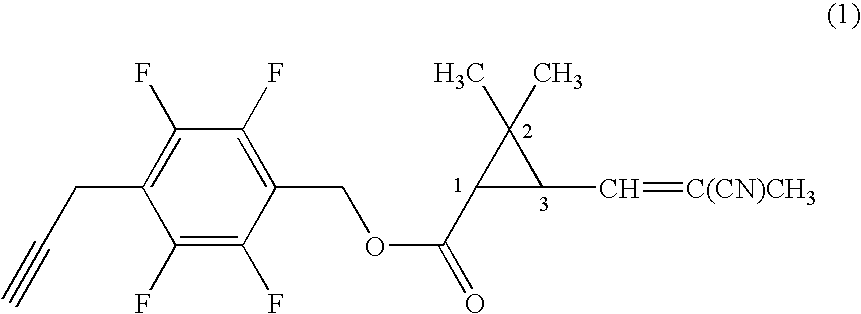
[0059]Under nitrogen atmosphere, to a mixture of 0.20 g of 4-propargyl-2,3,5,6-tetrafluorobenzyl alcohol, 0.17 g of (1R)-trans-3-(2-cyano-1-propenyl)-2,2-dimethylcyclopropanecarboxylic acid, 0.022 g of 4-dimethylaminopyridine in 7 ml of dichloromethane was added 0.21 g of N,N-dicyclohexylcarbodiimide, and the mixture was stirred at room temperature for three hours. Then, the reaction mixture was filtrated, and the filtrate was concentrated under reduced pressure. The resultant residue was subjected to silica gel column chromatography to obtain 0.26 g of 4-propargyl-2,3,5,6-tetrafluorobenzyl (1R)-trans-3-(2-cyano-1-propenyl)-2,2-dimethylcyclopropanecarboxylate (hereinafter, referred to as the present compound (A)) represented by the formula (A):
(an isomer ratio based on the double bond of the 2-cyano-1-propenyl group: Z / E=about 2 / 1).
[0060]1H-NMR (CDCl3, TMS) δ (ppm): 1.21 (s, 3H, Z+E forms), 1.32 (s, 3H, Z+E forms), 1.72 (t, 1H, Z+E forms), 1.96 (s, 3H, Z+E forms), 2.06 (s, 1H, Z+E
formulation example 1
[0062]To a solution of 20 parts of the present compound (A) in 65 parts of xylene is added 15 parts of Sorpol 3005X (registered trademark of Toho Chemical Industry Co., LTD.), and the mixture is thoroughly mixed with stirring to obtain an emulsifiable concentrate.
formulation example 2
[0063]To 40 parts of the present compound (A) is added 5 parts of Sorpol 3005X, and the mixture is thoroughly mixed with stirring. To the mixture are added 32 parts of Carplex #80 (synthetic hydrated silica, registered trademark of Shionogi & Co., Ltd.) and 23 parts of 300-mesh diatomaceous earth, and the resulting mixture is thoroughly mixed with a juice mixer to obtain a wettable powder.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap