Synthetic method for bifenazate
A technology of bifenazate and a synthesis method, applied in directions such as organic chemistry, can solve the problems of high impurity content of bifenazate, reducing the purity of bifenazate, poor removal effect of impurities, etc., and achieve the effect of improving purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
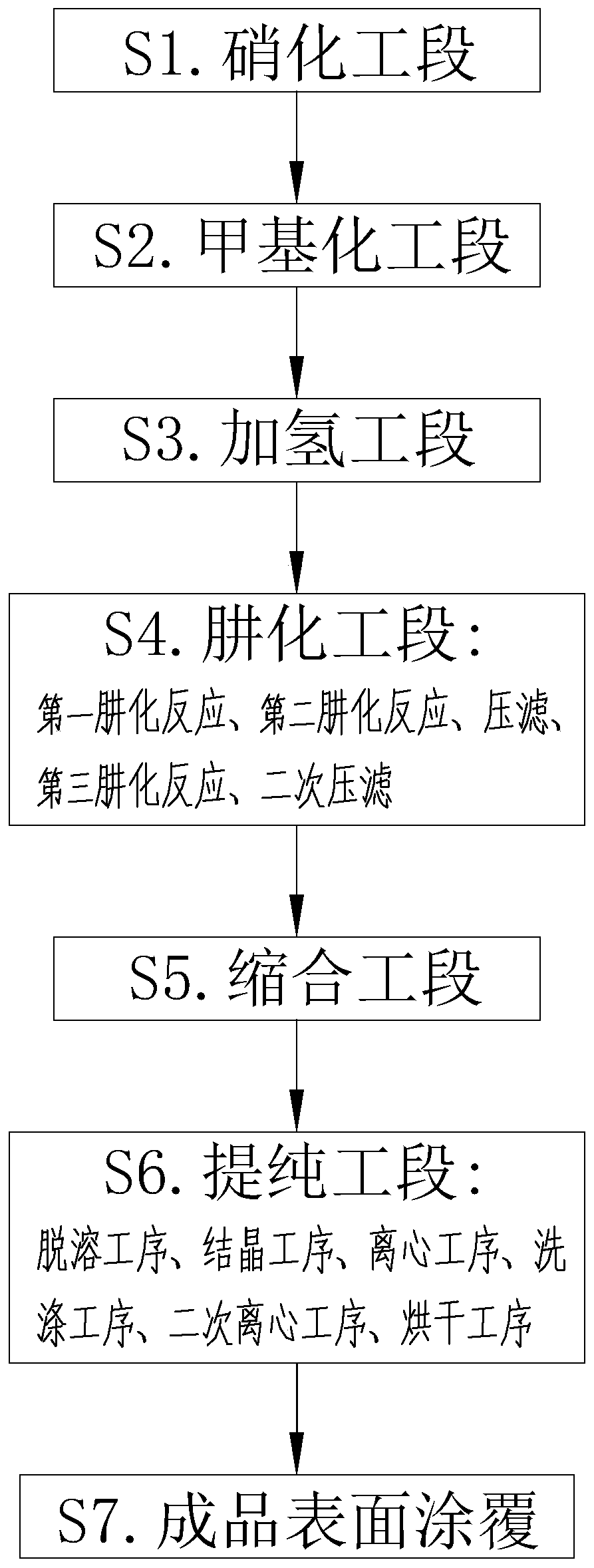
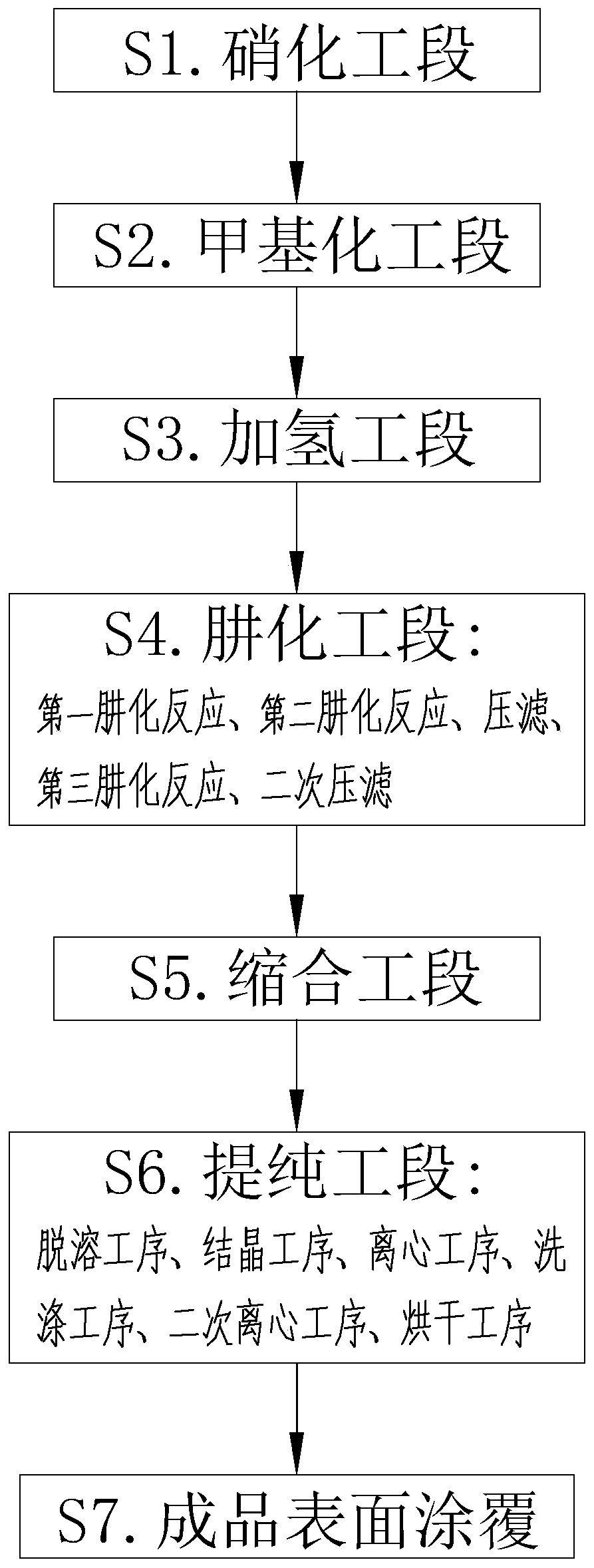
[0043] Embodiment 1 is a kind of bifenazate synthetic method disclosed by the present invention, and the specific synthetic section is as follows:
[0044] S1. Nitrification section: Mix and react 4-hydroxybiphenyl solution and toluene solution. The mass / number ratio of 4-hydroxybiphenyl solution and toluene solution is 1:4. After heating up to 70°C, gradually add HNO3 solution dropwise and keep warm React for 1h to obtain a nitration reaction solution;
[0045] S2. Methylation section: Mix the nitration reaction solution and anhydrous sodium carbonate powder for reaction, then add dimethyl carbonate solution dropwise, and keep it warm for 8 hours; add liquid alkali to the mixed solution, and remove water when the pH of the water layer is ≥ 11 layer, to obtain the methylation reaction solution;
[0046] S3. Hydrogenation section: inject hydrogen gas into the methylation reaction liquid, and react under the catalysis of Raney nickel, the reaction temperature is 55°C, and the re
Embodiment 2
[0062] Embodiment 2 is different from Embodiment 1 in that steps d, e, and f of S6 in Embodiment 1 are deleted.
Embodiment 3
[0063] Embodiment 3, the difference from embodiment 1 is to delete the d and e processes of S6 in embodiment 1, and directly put the first bifenazate filter cake produced in the c process into the double cone dryer in the f process Drying in medium to obtain finished product.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap