Method for synthesizing racecadotril
A racecadotril and synthesis method technology, applied in the field of synthesis of racecadotril, can solve the problems of difficult purification, high cost, high content, etc., and achieve the goals of reducing pollution, increasing production yield, and high atom utilization Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
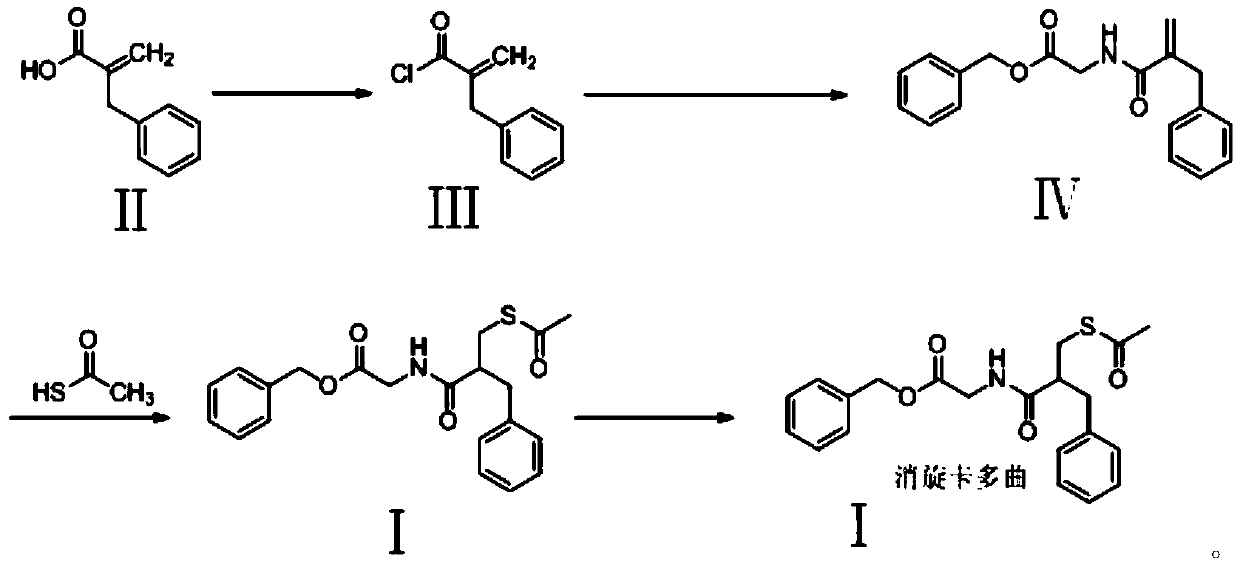
[0045] Example 1
[0046] 1) Preparation of 2-benzyl propionyl chloride (compound III)
[0047] In the 100L reactor, add 99.5kg methylene chloride, 15kg compound II and 0.15kg N,N-dimethylformamide, turn on stirring, cool down to 0°C, slowly add 14.1kg oxalyl chloride, finish adding, and heat up to 15 ℃, after holding the reaction for 2 hours, the above-mentioned reaction solution was concentrated under reduced pressure until almost no condensate flowed out to obtain 16.6 kg of light yellow oily liquid, yield: 99.4%
[0048] 2) Preparation of 2-de(acetylmercaptomethyl)-2-methylene racecadotril (compound IV)
[0049] 99.5kg of methylene chloride, 31.2kg of glycine benzyl ester p-toluenesulfonate and 28.1kg of triethylamine were added to the 300L reaction kettle. After stirring at room temperature for 30min, the temperature was cooled to 0°C, and compound III was slowly added. After the addition was completed, the mixture was stirred for 1h. Then, 45 kg of purified water was adde
Example Embodiment
[0060] Example 2
[0061] 1) Preparation of 2-benzyl propionyl chloride (compound III)
[0062] In the 100L reactor, add 100kg toluene, 15kg compound II and 0.15kg N,N-dimethylaniline, turn on stirring, be cooled to 10 DEG C, slowly add 15.5kg oxalyl chloride, finish adding, be heated to 25 DEG C, be incubated After 2 hours of reaction, the above reaction solution was concentrated under reduced pressure until no condensate flowed out to obtain 16.5kg of light yellow oily liquid, yield: 98.8%
[0063] 2) Preparation of 2-de(acetylmercaptomethyl)-2-methylene racecadotril (compound IV)
[0064] 99.5kg of dichloromethane, 18.6kg of glycine benzyl ester hydrochloride and 28.1kg of triethylamine were added to the 300L reactor, stirred at room temperature for 30min, cooled to 5°C, slowly added compound III, and after the addition was completed, after stirring for 1h, 45kg of purified water was added, stirred for 20min, left to stand for stratification, liquid separation, collected the
Example Embodiment
[0075] Example 3
[0076] 1) Preparation of 2-benzyl propionyl chloride (compound III)
[0077] Add 100kg of chloroform, 15kg of compound II and 0.15kg of pyridine to the 100L reaction kettle, turn on stirring, cool down to 10°C, slowly add 14.5kg of thionyl chloride, and after the addition, heat up to 25°C, keep the reaction for 2h, then add The above-mentioned reaction solution was concentrated under reduced pressure to substantially no condensate outflow to obtain 16.5kg of light yellow oily liquid, yield: 98.8%
[0078] 2) Preparation of 2-de(acetylmercaptomethyl)-2-methylene racecadotril (compound IV)
[0079] 99.5kg of dichloromethane, 18.6kg of glycine benzyl ester hydrochloride and 28.1kg of triethylamine were added to the 300L reactor, stirred at room temperature for 30min, cooled to 5°C, slowly added compound III, and after the addition was completed, after stirring for 1h, 45kg of purified water was added, stirred for 20min, left to stand for stratification, liquid s
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap