Application of carbon-supported ruthenium nanomaterial in preparation of N-alkyl aromatic amine compound
An alkyl aromatic amine and nanomaterial technology, which is applied in the field of catalytic chemistry, can solve the problems of inability to realize catalyst recycling, difficult separation of catalysts, low atom utilization rate, etc., and achieves high atom utilization rate, wide application range and uniform distribution. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0033] Example 1: Preparation of carbon-supported ruthenium nanomaterials
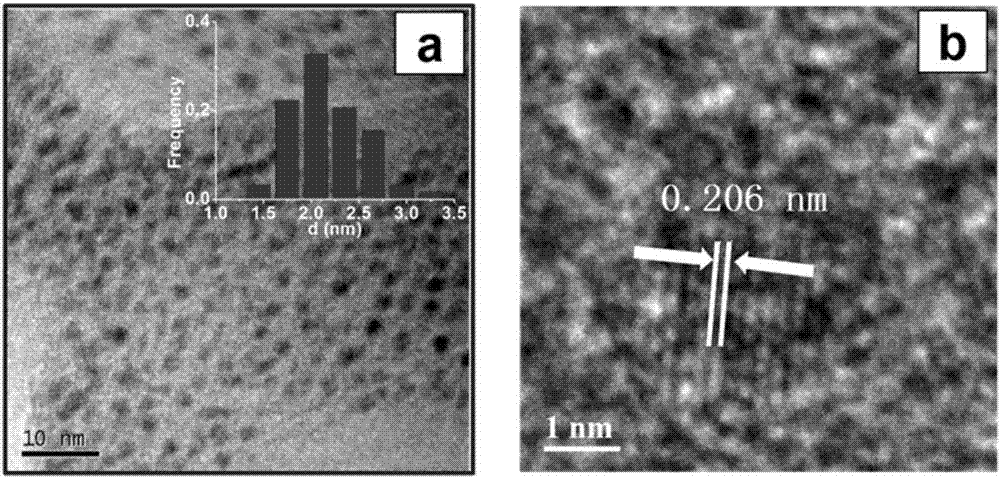
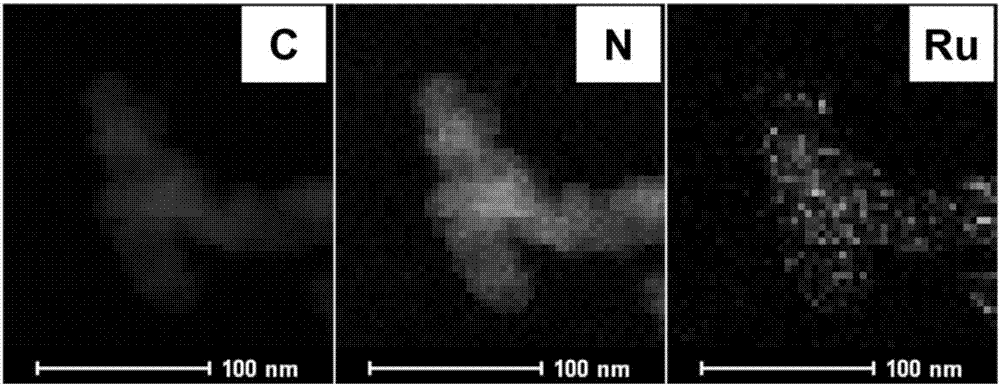
[0034] Put Ru(phen) 2 Cl 2 (0.0520 g), absolute ethanol (40 ml) was added to a 100 ml round bottom flask containing a magnetic stir bar, stirred at room temperature for 30 minutes, and then carbon material (VULCAN XC72R, 1.0000 g) was added to it, and reacted at 60°C 12 hours; after the reaction, the ethanol was removed by vacuum distillation under reduced pressure, and the solid was dried in an oven at 60°C for 12 hours. Then, put it in a magnetic boat and put it in a tube furnace. First, let nitrogen flow at room temperature for 30 minutes, then increase it from room temperature to 800°C at a rate of 5°C per minute, keep it for 2 hours, and then naturally cool to room temperature. The corresponding carbon-supported ruthenium nanomaterials are obtained, and the characterization is shown in the attachment Figure 1-5 ; Thermogravimetric analysis shows that the loading amount of ruthenium is 1.08% wt, ruthen
Example Embodiment
[0035] Example 2: Hydrogen transfer reaction of benzyl alcohol and aniline catalyzed by carbon-supported ruthenium nanomaterials
[0036]
[0037] Add carbon-supported ruthenium nanomaterials (20 mg) and potassium hydroxide (20 mg) into a 25 ml reaction tube with a branched opening equipped with a magnetic stirrer, repeatedly pump and fill with nitrogen three times, and add benzyl alcohol (1.3 mmol) through a syringe. ), aniline (1 mmol), toluene (3 ml) and then reacted for 24 h at 110°C under airtight; after the reaction, the catalyst was removed by filtration, water and ethyl acetate were added to extract the filtrate, the organic phases were combined, and dried , Filtration, concentration under reduced pressure, and purification by silica gel column chromatography to obtain N-benzylaniline (yield 90%).
[0038] The NMR data of the obtained product are as follows:
[0039] 1 H NMR (400 MHz, DMSO- d 6 , ppm) δ 7.36 (d, J = 7.3 Hz, 2H), 7.31 (t, J =7.4 Hz, 2H), 7.22 (t, J = 7.0 Hz,
Example Embodiment
[0043] Example 3: Hydrogen transfer reaction of 4-methylbenzyl alcohol and aniline catalyzed by carbon-supported ruthenium nanomaterials
[0044]
[0045] Add 4-methylbenzyl alcohol (1.3 mmol), carbon-supported ruthenium nanomaterials (20 mg), and potassium hydroxide (20 mg) into a 25 ml reaction tube with a branch with a magnetic stirrer, and repeatedly pump and fill with nitrogen Three times, add aniline (1mmol) and toluene (3ml) through a syringe, and then seal and react at 110°C for 24 hours; after the reaction, filter to remove the catalyst, add water and ethyl acetate to extract the filtrate, and combine the organic phases After drying, filtering, concentration under reduced pressure, and purification by silica gel column chromatography, N-(4-methylbenzyl)aniline was obtained (yield 92%).
[0046] The NMR data of the obtained product are as follows:
[0047] 1 H NMR (400 MHz, DMSO- d 6 , ppm) δ 7.23 (d, J = 8.0 Hz, 2H), 7.11 (d, J =7.8 Hz, 2H), 7.04–6.99 (m, 2H), 6.56 (d, J =
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap