Cobalt-free precursor for lithium ion battery, positive electrode material and preparation methods of cobalt-free precursor and positive electrode material
A technology for lithium-ion batteries and positive electrode materials, applied in battery electrodes, positive electrodes, secondary batteries, etc., can solve problems such as large fluctuations in Co prices and shortage of Co resources, and achieve accelerated deintercalation, poor rate performance, and high The effect of specific capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
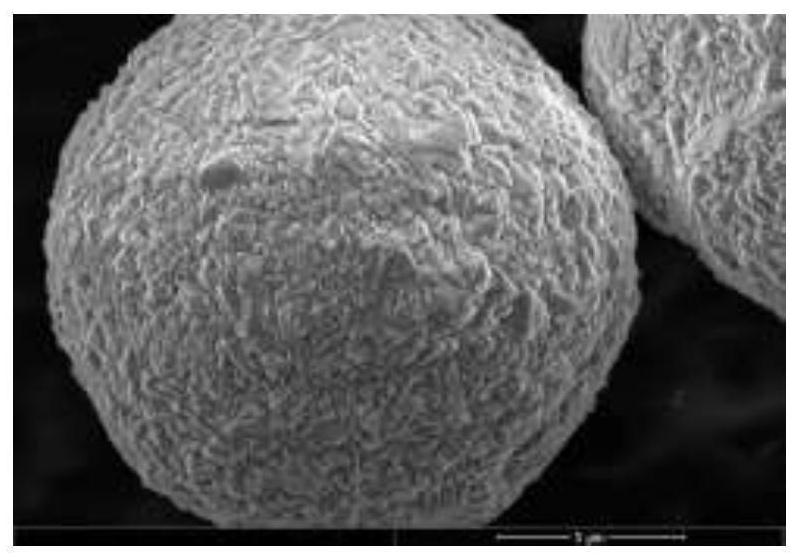
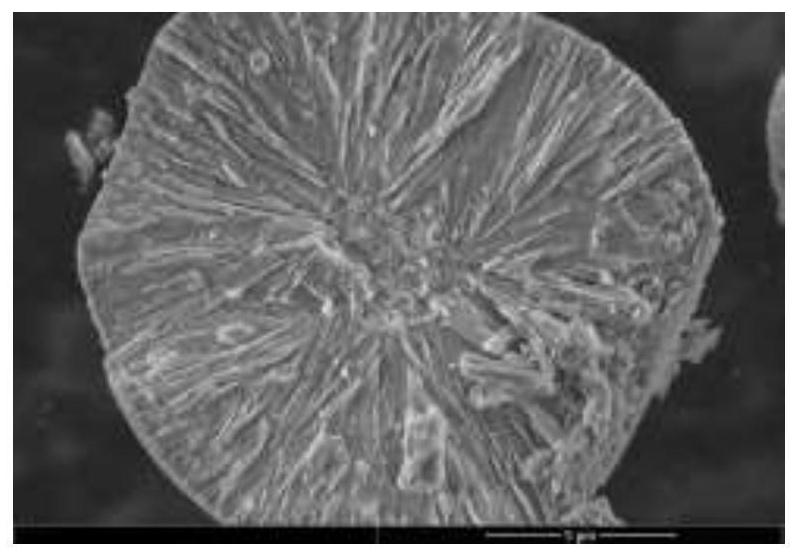
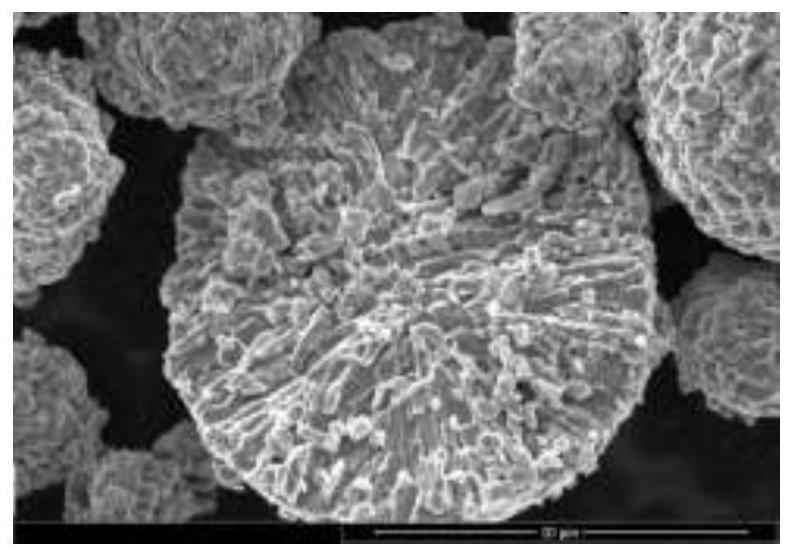
Image
Examples
Embodiment 1
[0024] Step 1: nickel sulfate and manganese sulfate are configured into A solution according to the molar ratio Ni:Mn=80:20, nickel sulfate and manganese sulfate are configured into B solution according to the molar ratio Ni:Mn=60:40, A solution, B solution The total concentration of nickel and manganese in the medium is 2.5mol / L.
[0025] Step 2: Add 300L of pure water, 3kg of liquid caustic soda and 40kg of ammonia water into the reaction kettle, the ammonia concentration of the bottom liquid is 5g / L, and the pH is 12.0. 1m into the reactor 3 / h of inert gas to prevent the oxidation of Mn in the reaction process, and then use a metering pump to add A solution to the reactor with a flow rate of 30kg / h. The flow rate is added to the reactor for stirring, and the stirring rate is 300rpm / min. Among them, the concentration of the added ammonia water is 7g / L, and the concentration of the added liquid caustic soda is 2.5mol / L. During the reaction process, the flow rate
Embodiment 2
[0030]Step 1: Nickel sulfate and manganese carbonate are configured into A solution according to the molar ratio Ni:Mn=85:15, nickel sulfate and manganese carbonate are configured into B solution according to the molar ratio Ni:Mn=70:30, A solution, B solution The total concentration of nickel and manganese in the medium is 3mol / L.
[0031] Step 2: Add 300L of pure water, 2.75kg of liquid caustic soda and 45kg of ammonia water into the reaction kettle, the ammonia concentration of the bottom liquid is 7g / L, and the pH is 11.8. 1m into the reactor 3 / h of inert gas to prevent the oxidation of Mn in the reaction process, and then use a metering pump to add solution A to the reactor at a flow rate of 60kg / h. Add reactor and stir, stirring rate is 250rpm / min, and wherein, the concentration of the ammoniacal liquor that adds is 9g / L, and the concentration of the liquid caustic soda that adds is 4mol / L, by controlling the flow rate of ammoniacal liquor and liquid caustic
Embodiment 3
[0036] Step 1: nickel carbonate and manganese carbonate are configured into A solution according to the molar ratio Ni:Mn=91:09, nickel carbonate and manganese carbonate are configured into B solution according to the molar ratio Ni:Mn=75:25, A solution, B solution The total concentration of nickel and manganese in the medium is 3.5mol / L.
[0037] Step 2: Add 300L of pure water, 2.5kg of liquid caustic soda and 47kg of ammonia water into the reactor to maintain the pH of the bottom liquid at 11.6 and the ammonia concentration at 8g / L. In order to pass 1m into the reactor 3 / h of inert gas to prevent the oxidation of Mn in the reaction process, and then use a metering pump to add solution A to the reactor at a flow rate of 50kg / h. Add the reaction kettle to stir, the stirring rate is 200rpm / min, wherein, the concentration of the added ammonia water is 14g / L, the concentration of the added liquid caustic soda is 4.5mol / L, adjust the system by controlling the flow of
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap