METHODS FOR USING ALDHbr CELLS TO SUPPLEMENT STEM CELL TRANSPLANTATION
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Immune Reconstitution after Unrelated Mismatched UCB Transplantation
[0055]Immune reconstitution has been evaluated in approximately 100 survivors of UCB transplantation that have been followed for a median of 650 days (range 121-2450 days). The results of this study can be found in Klein et al. (2001) Biol Blood and Marrow Trans 7:454-466. Briefly, functional and immunophenotypic parameters were assayed in engrafted patient's peripheral blood at 3, 6, 9, 12, 24, and 36 months post transplant. Patients were generally maintained on methyprednisolone for the first three months post transplant and cyclosporine for the first year post transplant. Immunizations were reinstituted in the second and third years post transplant. All surviving patients without active chronic GvHD received the full complement of killed and live vaccines per the usual CDC recommendations. Infants and toddlers <2 years of age recovered T-cell immune function as measured by CD4 counts and PHA responses by 6 months po
example 2
Clinical Results of UCB Transplantation in Pediatric Patients with Inborn Errors of Metabolism
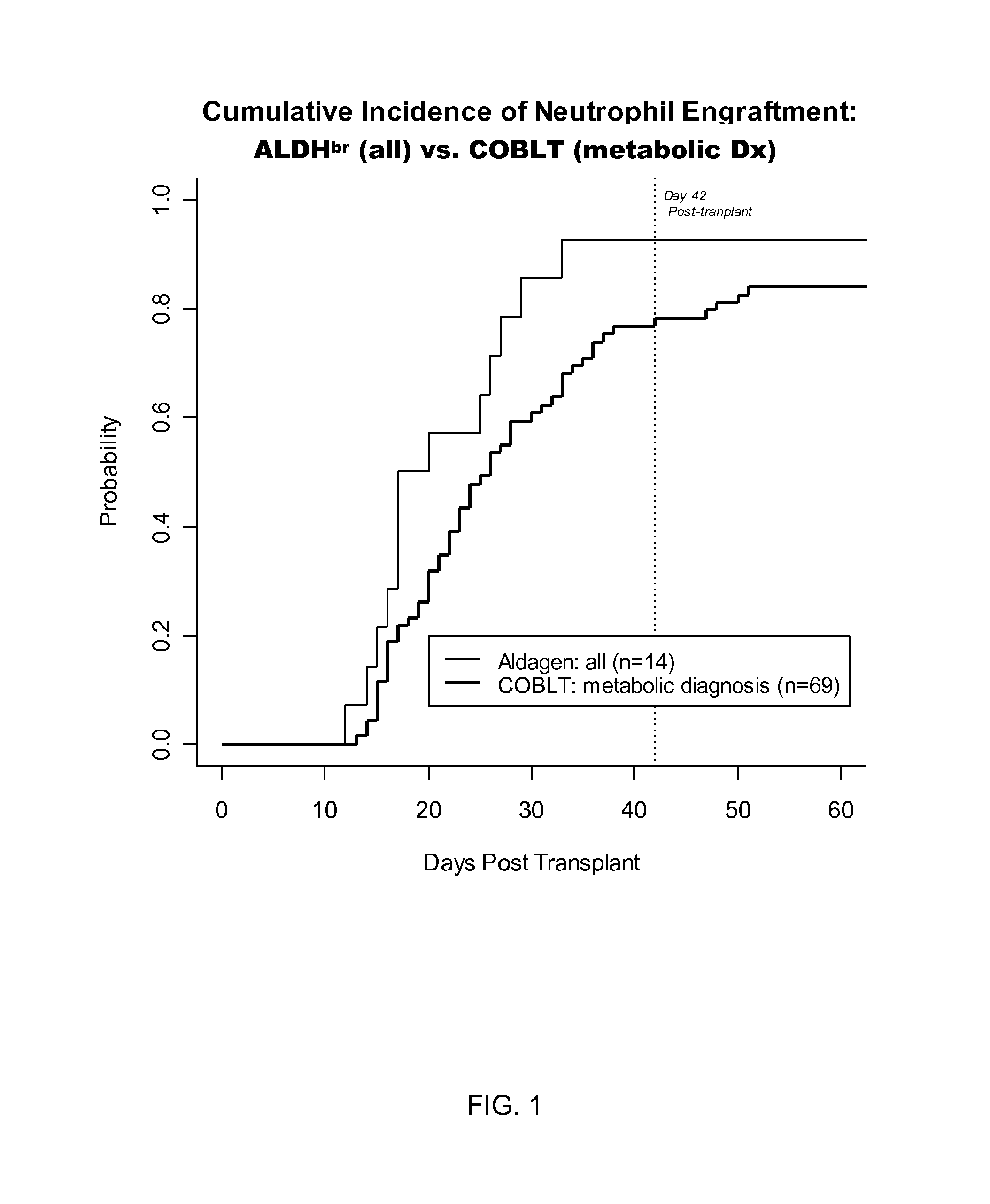
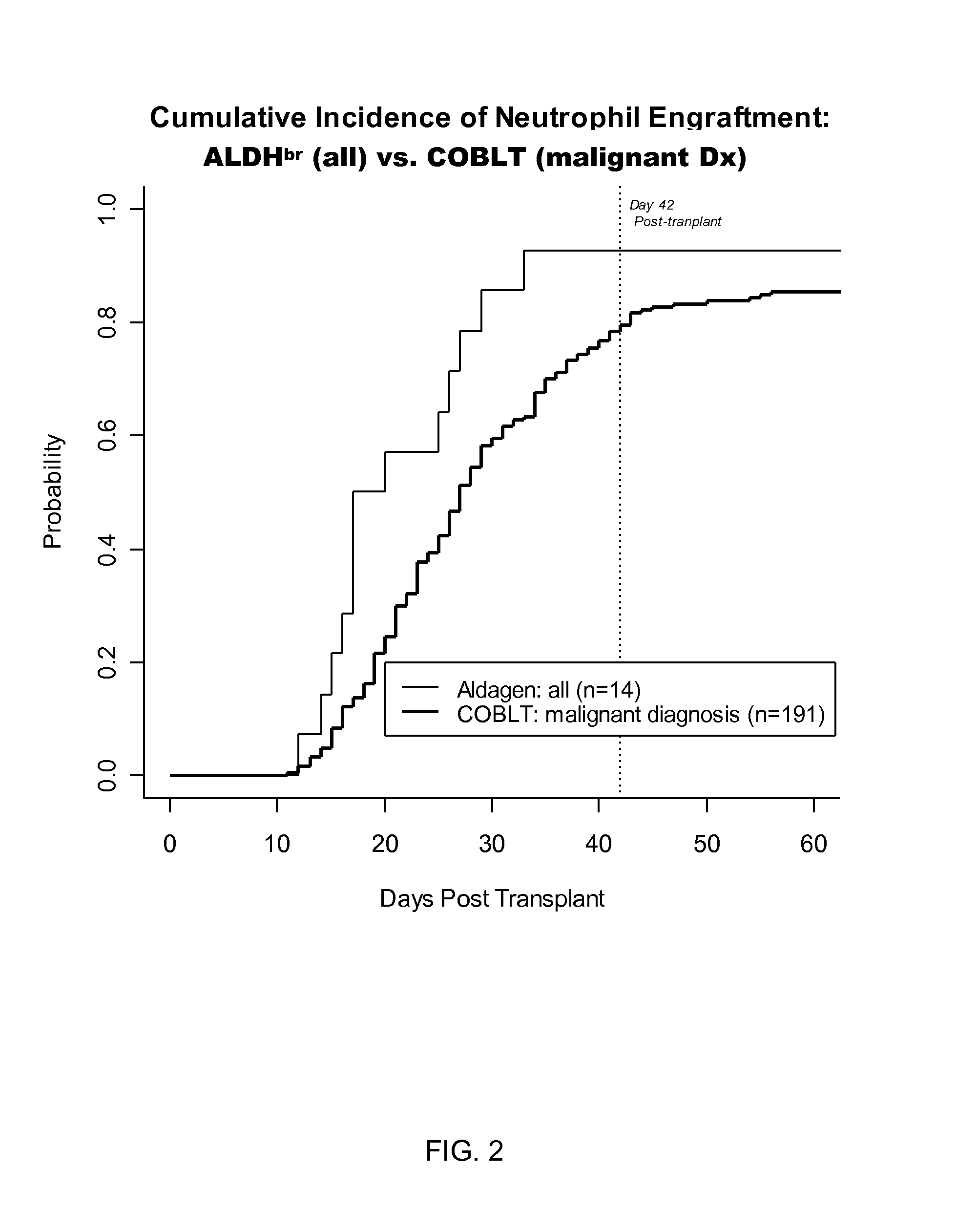
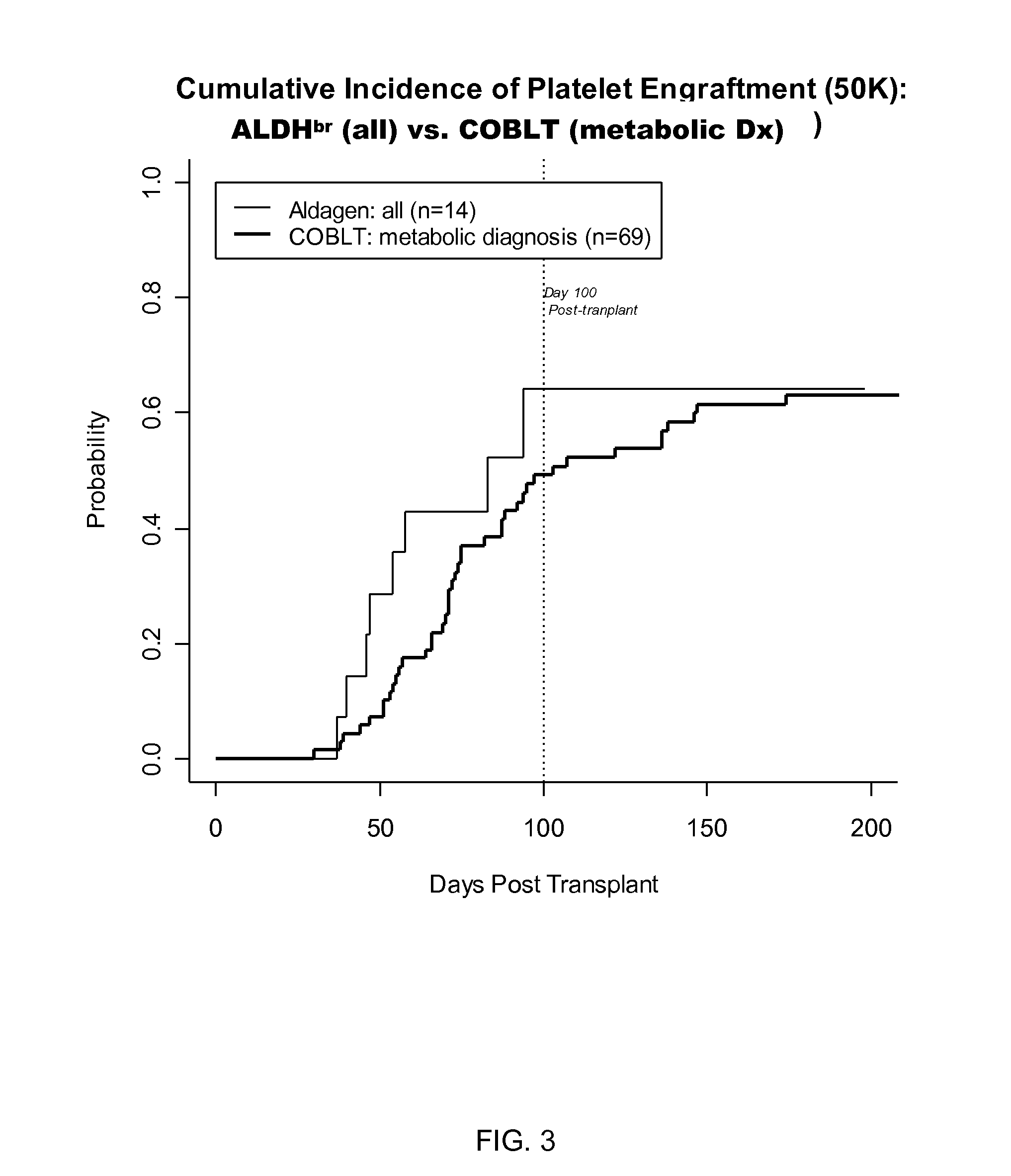
[0056]Recent results from the Cord Blood transplantation Study (COBLT), a multi-institutional, prospective NIH-sponsored trial of unrelated donor cord blood transplantation have further advanced the field of UCBT. See, Kurtzberg et al. (2005) Biology of Blood and Marrow Transplantation 11(2):2 (abst 6); Kurtzberg et al. (2005) Biology of Blood and Marrow Transplantation 11(2):82(Abst 242).
[0057]A different strata of the COBLT study evaluated the efficacy of cord blood transplantation in 69 children with inborn errors of metabolism, augmenting prior and pending reports results of UCBT in babies with Infantile Krabbe Disease and Hurler Syndrome (MPS I). A common protocol was used for the preparative regimen (busulfan, cyclophosphamide, ATG) and GvHD prophylaxis (cyclosporine, steroids). Patients with MPS 1-V (n=36, 20 previously reported), globoid cell leukodystrophy (Krabbe Disease, n=16), adre
example 3
Prepurification Steps to Enrich for ALDHbr UCB Cells
[0059]The cord blood unit selected for transplantation was stored in a 2 compartment cryopreservation bag (20% / 80% split) in a total of 25 ml of cells, hespan and 10% DMSO. On day −5 before transplant, the 20% (5 ml) fraction was removed from liquid nitrogen (procedure 5D.160.01), and thawed in a 37 degree C. waterbath to a slushy consistency. Dextran / Albumin was added to dilute to 4× the initial volume, the cells were washed, pelleted and resuspended in ALDESORT® assay buffer / 100 U / ml DNase I (Aldagen, Inc., Durham, N.C.). Red blood cell to white blood cell ration was adjusted to <1×10e8 cells / ml and the cells were lineage depleted with EASYSEP® (StemCell Technologies) anti-glycophorin A and CD14 cocktails to label cells. The labeled cells were mixed with EASYSEP® magnetic nanoparticles and incubated at room temperature for 10 minutes. The sample was then exposed to the EASYSEP® magnetic which will remove lineage positive c
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap