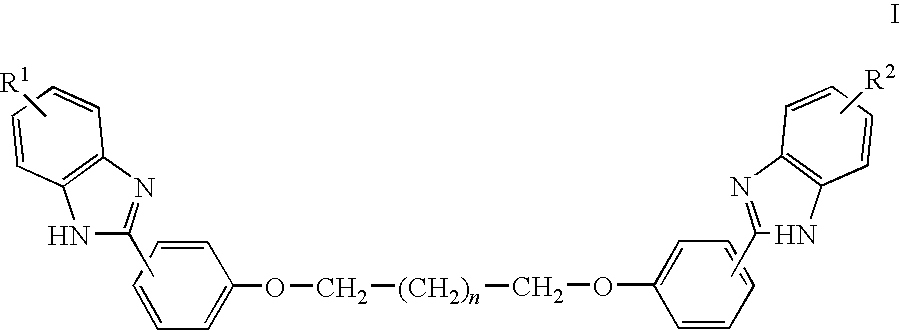Bisbenzimidazoles as antimalarial agents
a technology of bisbenzimidazole and antimalarial drugs, which is applied in the field of bisbenzimidazole, can solve the problems of increasing the resistance of many of the currently available antimalarial drugs, threatening people in areas where malaria is prevalent, and failing to develop vaccines against i>p. falciparum /i>,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0072]The following general procedures and intermediates were used to prepare the compounds described herein. Compounds for use in the present invention can be prepared as described in Scheme 1.
with n=0 to 10, and each R is independently selected from hydrogen, halogen, hydroxyl, alkyl, alkoxy, amino, alkylamino, alkylcarbonylamino, alkylcarbonyloxy, formyl, alkylcarbonyl, alkyloxycarbonyl, halocarbonyl, haloalkyl, haloalkoxy, carbamoyl, cyano, nitro, sulfo, or a carboxyl group.
[0073]For example, each R is independently selected from H, —CO2H, Cl, F, Br, —OH, -Me, -Et, -nPr, -iPr, tBu, —OMe, —OEt, —OnPr, —OiPr, —OnBu, —OiBu, —OtBu, —O—C5—H11, —O—C6H13, —O—C7H15, —O—C8H17, —O—C9H19, —O—C10H21, —O—C11H23, O—C12H25, —O—C13H27, —O—C14H29, . . . , —OC18H37, . . . —OC24H49, —NH2, —NH(Me), —N(Me)2, —N(Et)2, —NH—CO-Me, —NH—CO-Et, —OCOMe, —OCOEt, —OCOnPr, —OCOiPr, —OCOnBu, —OCOiBu, —OCOtBu, —OCO—C5H11, —OCO—C6H13, —OCO—C7H15, —OCO—C8H17, —OCO—C9H19, —OCO—C10H21, —OCO—C11H23, OCO—C12H25, —OCO—C1
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
- R1 and R2 are each independently selected from hydrogen, halogen, hydroxyl, alkyl, alkoxy, amino, alkylamino, alkylcarbonylamino, alkylcarbonyloxy, formyl, alkylcarbonyl, alkyloxycarbonyl, halocarbonyl, haloalkyl, haloalkoxy, carbamoyl, cyano, nitro, sulfo, or a carboxyl group.
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap