Ordered flagellin array as an immunostimulant
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
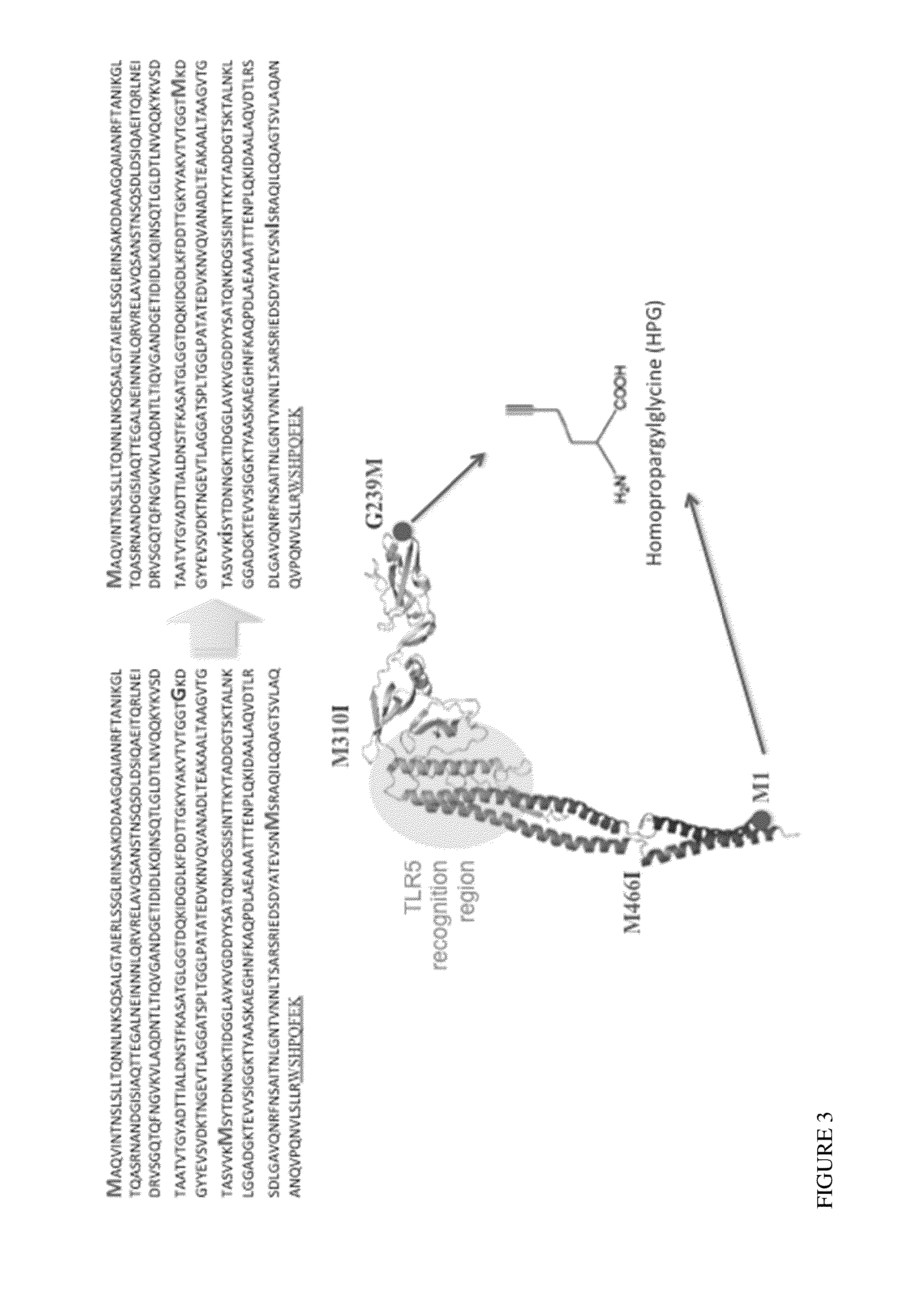
[0097]Ordered flagellin array as an immunostimulant. Immune-stimulation by bacterial flagellins has been explored as a potential vaccine adjuvant both for induction of specific humoral and cellular immune responses. Flagellin monomers (FIG. 1) have four globular domains (D0, D1, D2 and D3), of which the D0 and D1 domains are the most highly conserved. Recent studies suggest that the conserved D1 and D2 domains are important for innate immune system stimulation.
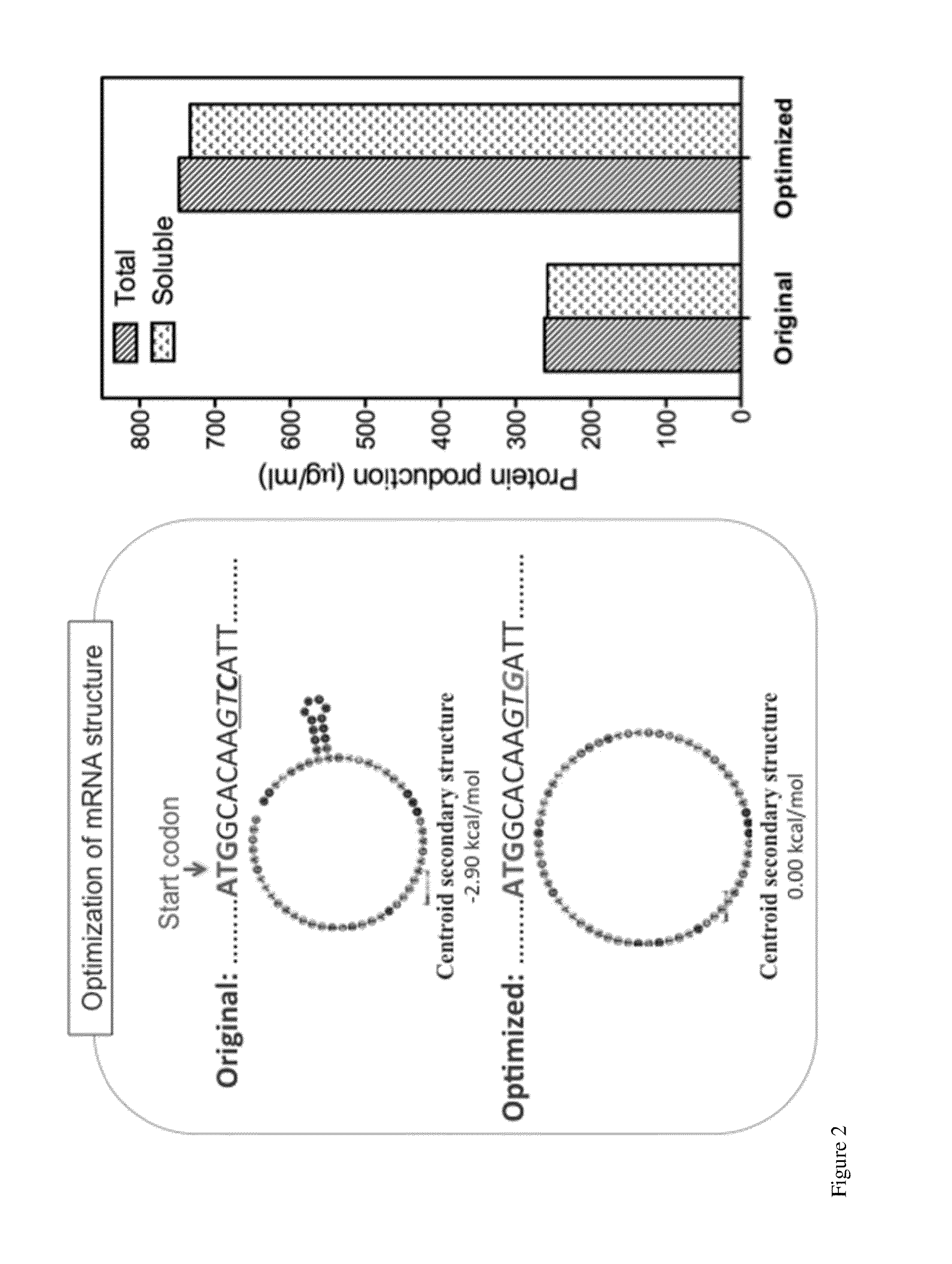
[0098]Cell-free protein synthesis (CFPS) provides a means for rapid production of flagellin protein. We cloned the flagellin gene (flic) from Salmonella typhimurium SL1344. The protein yield was 260 μg / ml. To increase the yield, we optimized the mRNA secondary structure of the first 40 nucleotides. By the mutation of only one nucleotide, the protein yield increased to 750 μg / ml, as shown in FIG. 2.
[0099]Virus-like particles (VLPs) can elicit strong immune response. We sought to attach flagellin protein to VLPs in order to pre
example 2
[0115]Bacterial flagellin has been explored as a potential vaccine adjuvant for inducing innate immunity. We recently developed an Escherichia coli-based cell-free protein synthesis (CFPS) method to rapidly produce soluble flagellin protein. Observations from protein purification and SDS-PAGE indicated that the C-terminal D0 domain of flagellin is susceptible to proteolytic degradation. This proteolysis could be reduced by protease inhibitors. The addition of the protease inhibitor cocktail both increased the yield of flagellin (FIG. 6(a)) and avoided the appearance of the truncated product (FIG. 6(b)).
[0116]Since E. coli BL21 is deficient in the Lon and OmpT proteases, we compared BL21 cell extract with KC6 extract in the CFPS system, as shown in FIG. 7. Autoradiography indicated no truncated flagellin accumulation using BL21 extract. However, the total yield using BL21 extract (123.5 μg / mL) was much lower than that using KC6 extract (336.3 μg / mL). To increase the CFPS yield using
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
| Efficiency | aaaaa | aaaaa |
| Affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap