Pharmaceutical formulations for sustained release of sebacoyl dinalbuphine ester
a technology of dinalbuphine and formulation, applied in the field of pharmaceutical compositions, can solve the problems of serious injection site irritation, time-consuming and uncost effective large-scale production, and inconvenient administration of supensions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
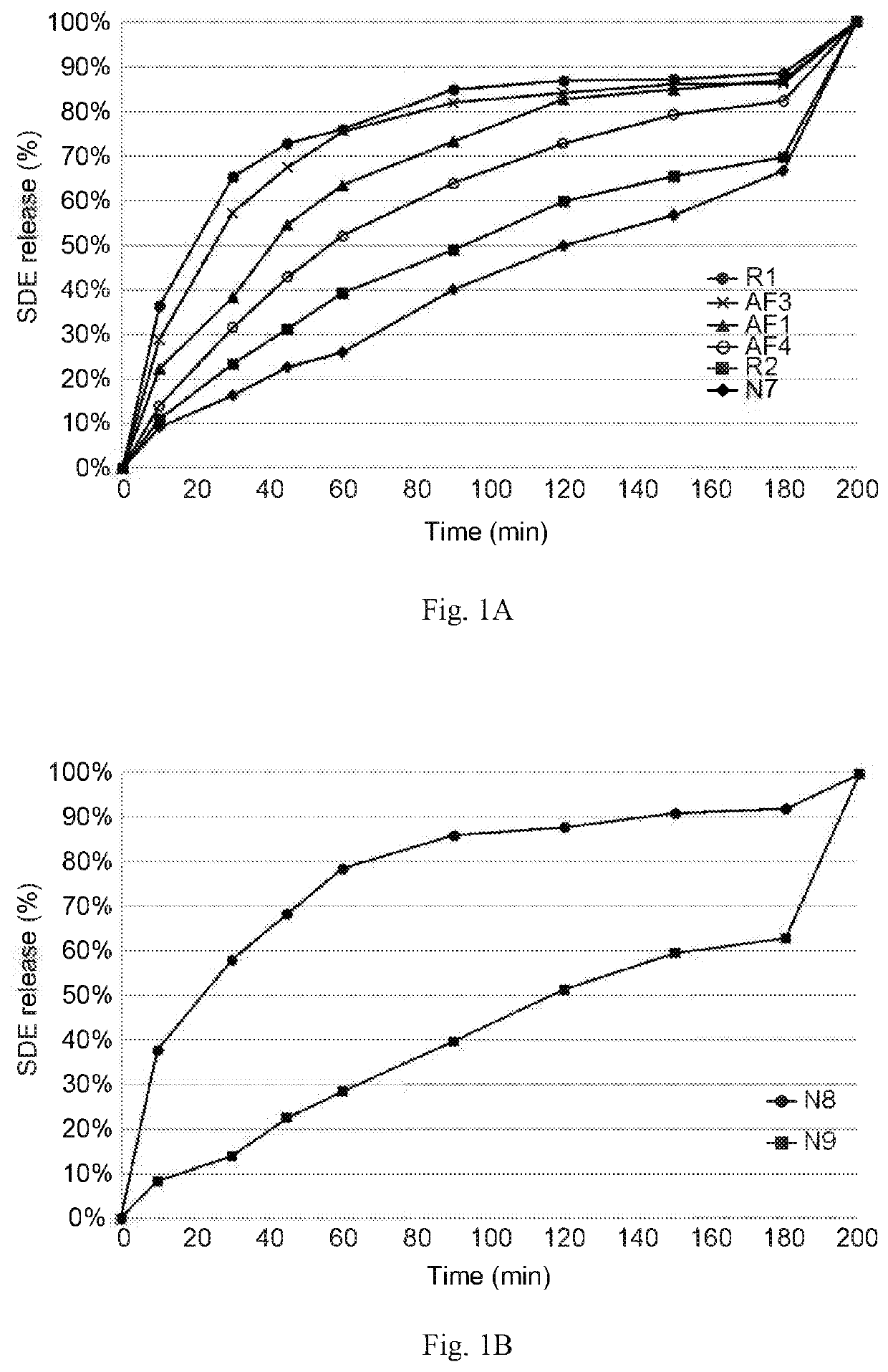
Release Study of Present Formulations with Various BB / Oil Ratio
[0141](1) Preparation of the Present Formulations
[0142]Eight present formulations with various SDE concentrations (50-150 mg / mL) and various weight ratios of benzyl benzoate to sesame oil (“BB / oil ratio”), or the mixture of benzyl benzoate and benzyl alcohol to sesame oil (“BB+BA / oil ratio”) (0.5-19) were prepared based on Table 1A and Table 1B. Among them, AF4, R2, N7 and N9 formulations were prepared according to the following Method A.
[0143]Method A:
[0144]The solvent systems (i.e., the mixture of benzyl benzoate, with or without benzyl alcohol, and sesame oil) were respectively prepared by mixing each component with the predetermined volume corresponding to the desired weight / weight percent (w / w %) listed in Table 1A and Table 1B. The resulting solvent mixtures were vortexed or stirred at room temperature to fully mix each component. The predetermined amounts of SDE were respectively weighed, based on the SDE
example 3
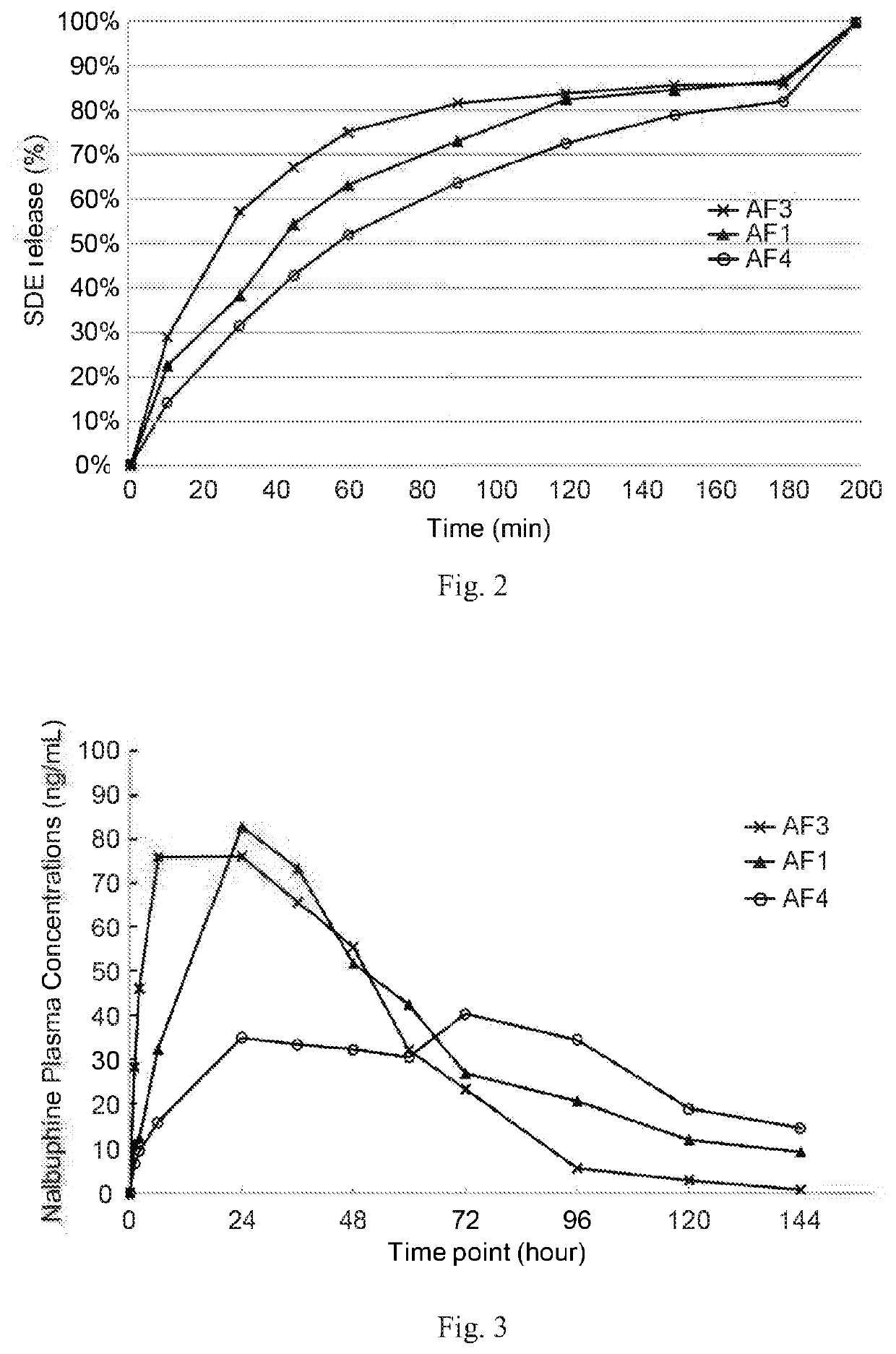
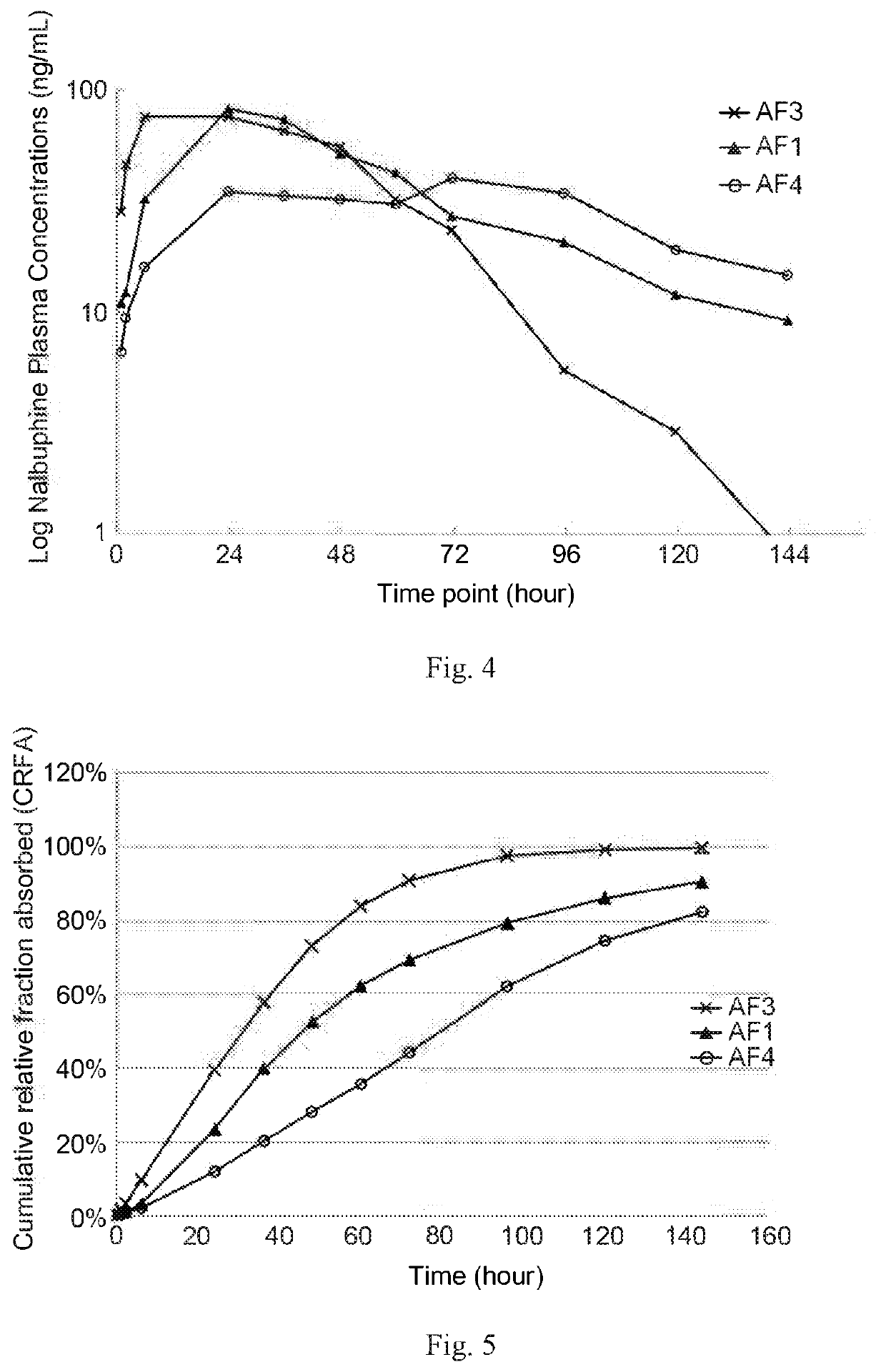
and In Vivo (on Dogs) Studies of Present Formulations
[0175](1) Preparation of Present Formulations
[0176]Three SDE formulations (AF3, AF1, and AF4) were prepared based on the concentrations and BB / oil ratios listed in Table 3. AF4 was prepared according to Method A of Example 1; AF3 and AF1 were prepared according to Method B of Example 1.
TABLE 3Present formulations for in vitro and in vivo studies on dogsSampleBenzyl benzoateSesame oilSDEBB / oilNo.(w / w %)(w / w %)(mg / mL)ratioAF33961500.65AF154.245.8801.18AF46733802
[0177](2) Stability of Present Formulation
[0178]AF3, AF1 and AF4 formulations were then subject to a freeze-thaw test to check its physical stabilities. The freeze-thaw test was conducted by cooling each of the samples at about 0-4° C. for about 12 hours, warming each of the cooled samples at room temperature for about 12 hours, and sequentially repeating the cooling and warming steps twice. AF3, AF1, and AF4 formulations stayed clear and homogeneous after the freeze-t
example 4
and In Vivo (on Humans) Studies of Present Formulations
[0193](1) Preparation of Present Formulations, by Method B of Example 1
[0194]About 600 g of SDE was mixed with about 4025 g of benzyl benzoate. The resulting mixture was stirred at 300 rpm for 60 minutes to give a clear solution. About 3591 g of sesame oil was added into the clear solution, and then stirred at 300 rpm for about 30 minutes. The resulting solution was subjected to filtration sterilization by using Millipore 0.22 μm filters. The final formulation (F8) had an SDE concentration of about 75 mg / mL, and the weight ratio of benzyl benzoate to sesame oil was about 1.12:1 (Table 6).
TABLE 6F8 formulationSampleBenzyl benzoateSesame oilSDEBB / oilNo.(w / w %)(w / w %)(mg / mL)ratioF85347751.12
[0195]The F8 formulation thus obtained was a homogeneous solution without solid particles, thereby suitable for being sterilized directly by filtration and suitable for large scale production.
[0196](2) In Vitro Dissolution Experiment
[0197
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Magnetic field | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap