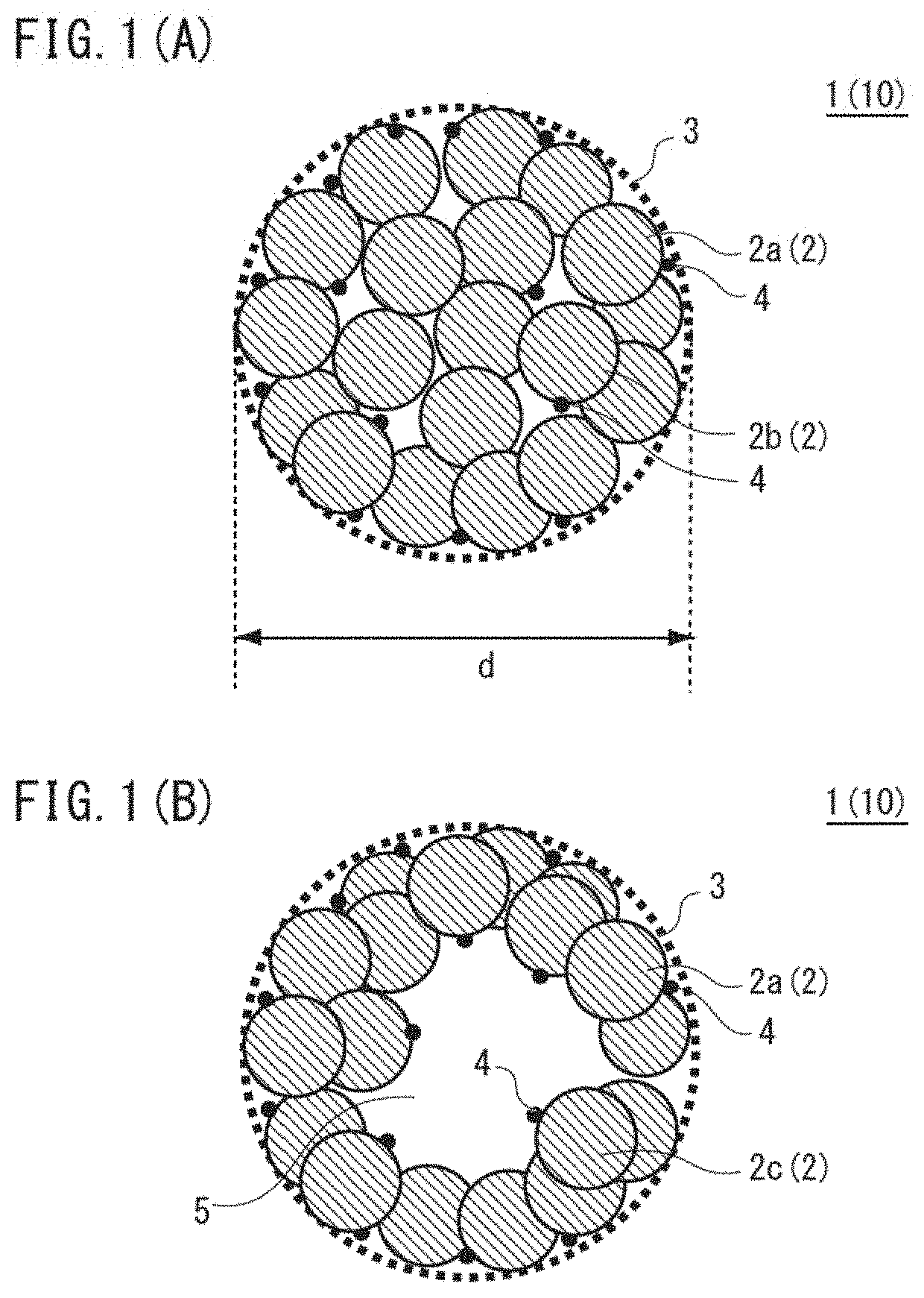
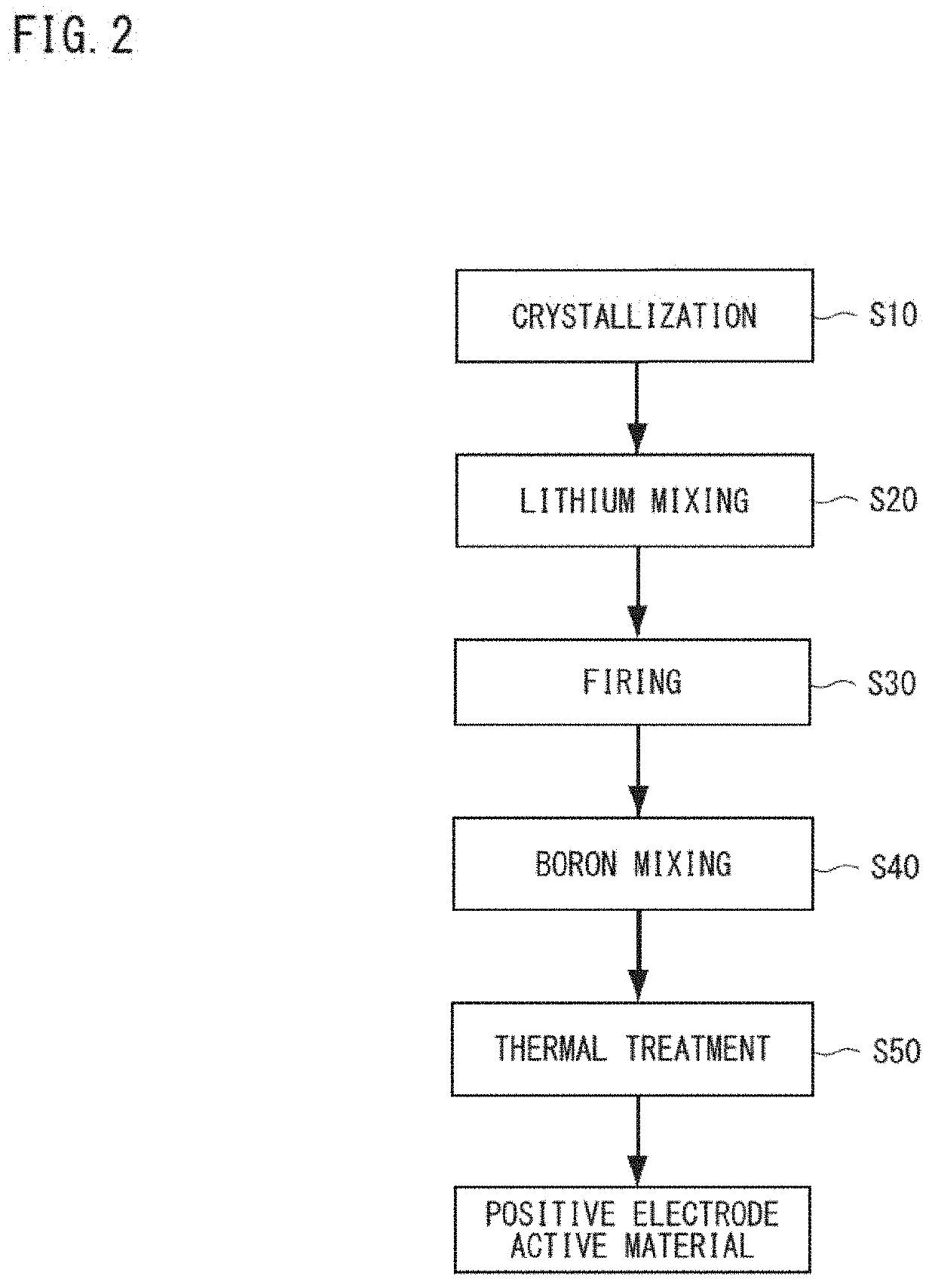
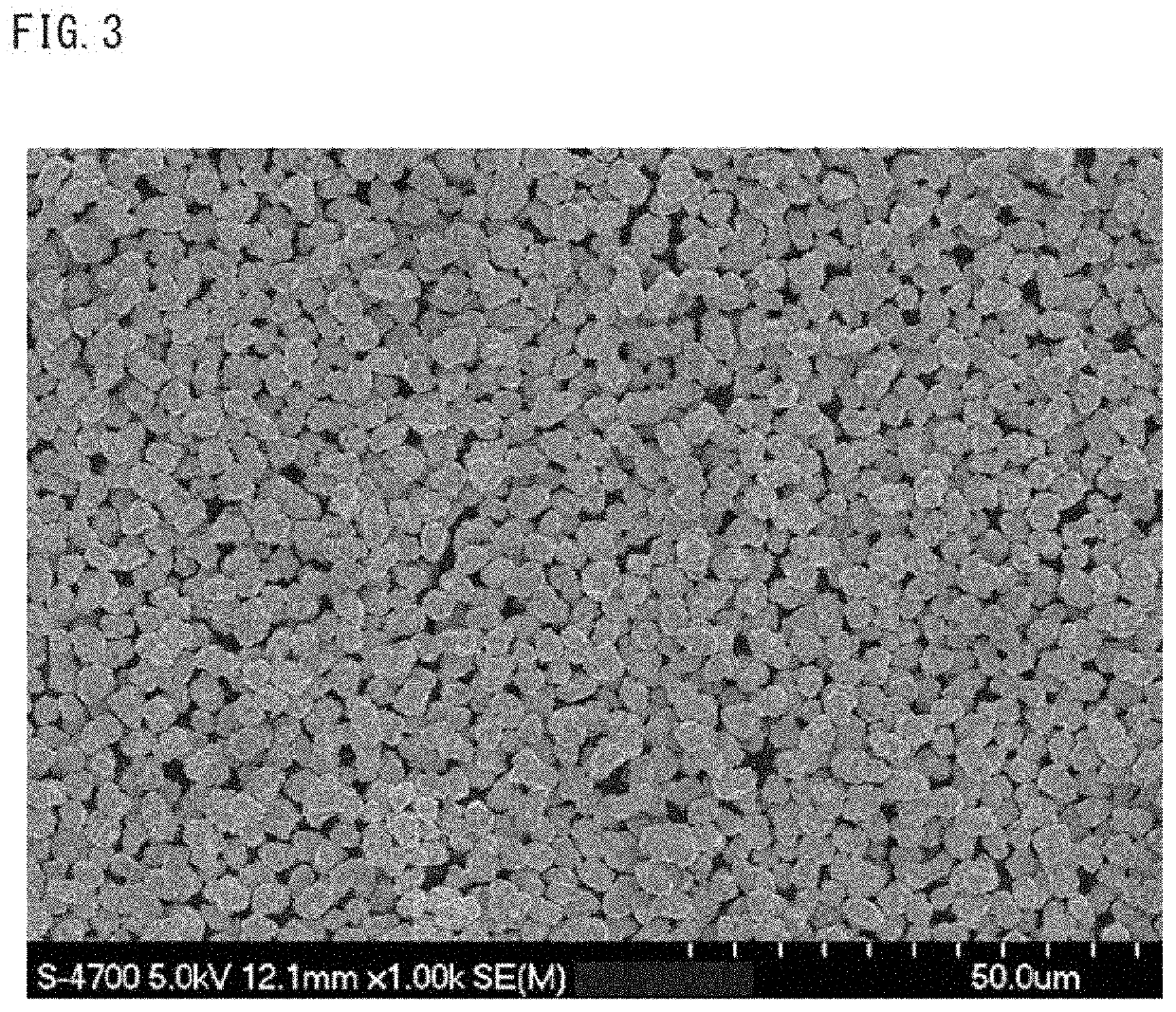
Positive electrode active material for nonaqueous electrolyte secondary batteries, method for producing same and nonaqueous electrolyte secondary battery using said positive electrode active material
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Example
[0057]As shown in Comparative Example 5 in FIGS. 5(A) and 5(C), for example, when the positive electrode active material 1 excessively contains boron, the water-soluble Li amount may increase (FIG. 5(C)), and the intimal discharging capacity may sharply reduce (FIG. 5(A)). As described above, it is considered that lithium within the crystals of the lithium-metal composite oxide particles (raw materials) is excessively extracted, the number of lithium ions contributing to charging and discharging reduces, and thus battery capacity reduces.
[0058]The lithium-metal composite oxide 1 has a hexagonal layered crystal structure, and its crystallinity can be evaluated with the length of a c-axis (hereinafter, also referred to as a “c-axis length”) obtained by performing Rietveld analysis on an X-ray diffraction result or a lithium seat occupancy (hereinafter, may be referred to as “Li seat occupancy”) in lithium sites within crystals, for example.
[0059]The Li seat occupancy of the lithium-metal
Example
Example 1
[0153](Crystallization Process)
[0154]First, water was put into a reaction tank (60 L) to fill it halfway and was stirred in the atmosphere, and an intra-tank temperature was set to 40° C., to which appropriate amounts of a 25% by mass aqueous sodium hydroxide solution and 25% by mass ammonia water were added. The pH value of the liquid within the tank was adjusted to 12.8 with a liquid temperature of 25° C. as a basis, and the ammonia concentration within the liquid was adjusted to 10 g / L. Added thereto was a 2.0 mol / L mixed aqueous solution of nickel sulfate, cobalt sulfate, and manganese sulfate (with a metal element molar ratio of Ni:Co:Mn=38:32:30) at a rate of 130 ml / minute to obtain a reaction aqueous solution. At the same time, 25% by mass ammonia water and a 25% by mass aqueous sodium hydroxide solution were added thereto at a constant rate to perform crystallization for 2 minutes and 30 seconds while controlling the pH value to 12.8 (nucleation pH). Subsequently
Example
Example 2
[0162]A positive electrode active material was obtained and evaluated similarly to Example 1 except that the heat treatment temperature during the addition of boric acid was changed to 210° C. Table 1 and Table 2 list evaluation results.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap