Improved method for purification of immunoglobulin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
bulin Purification
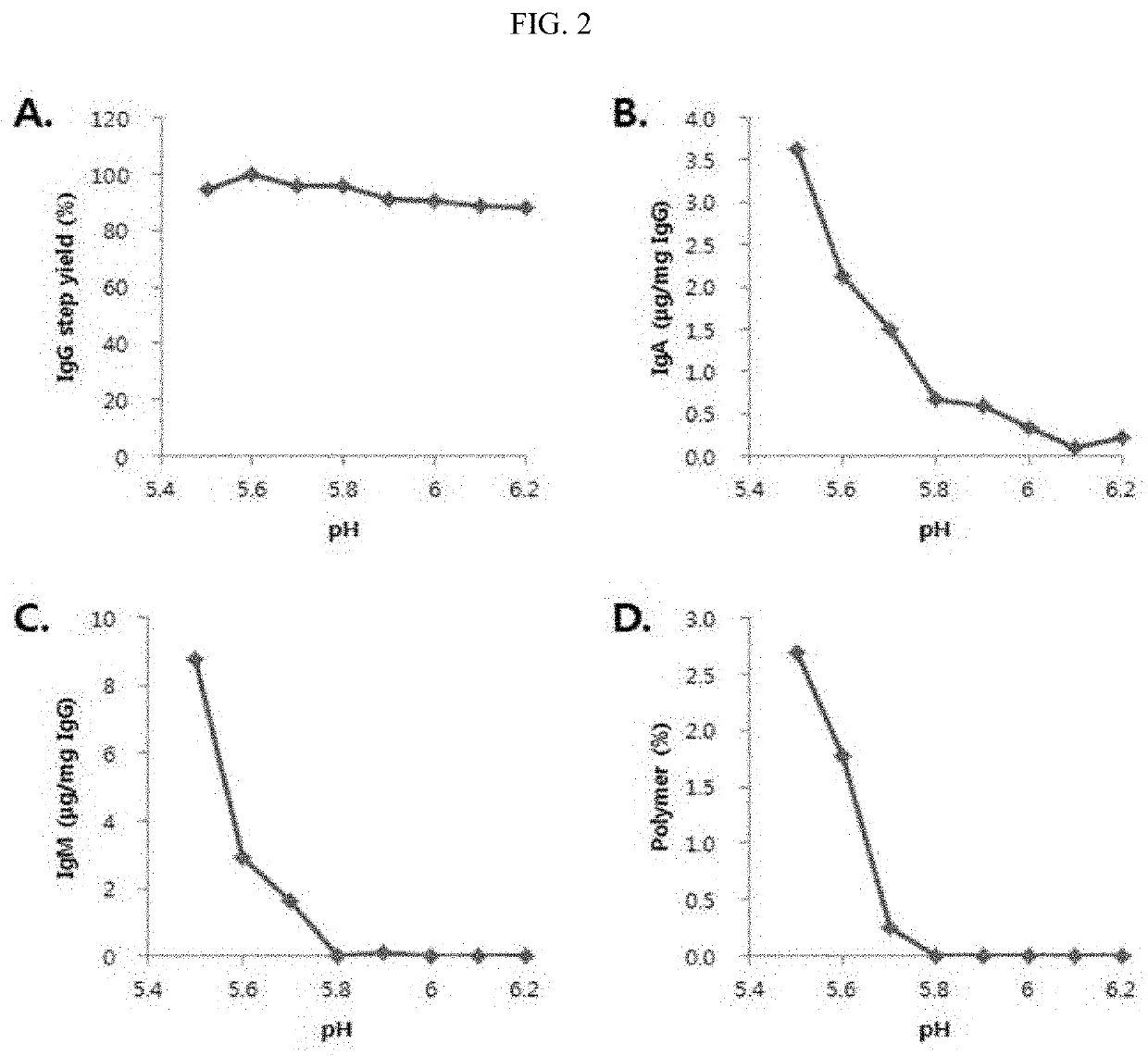
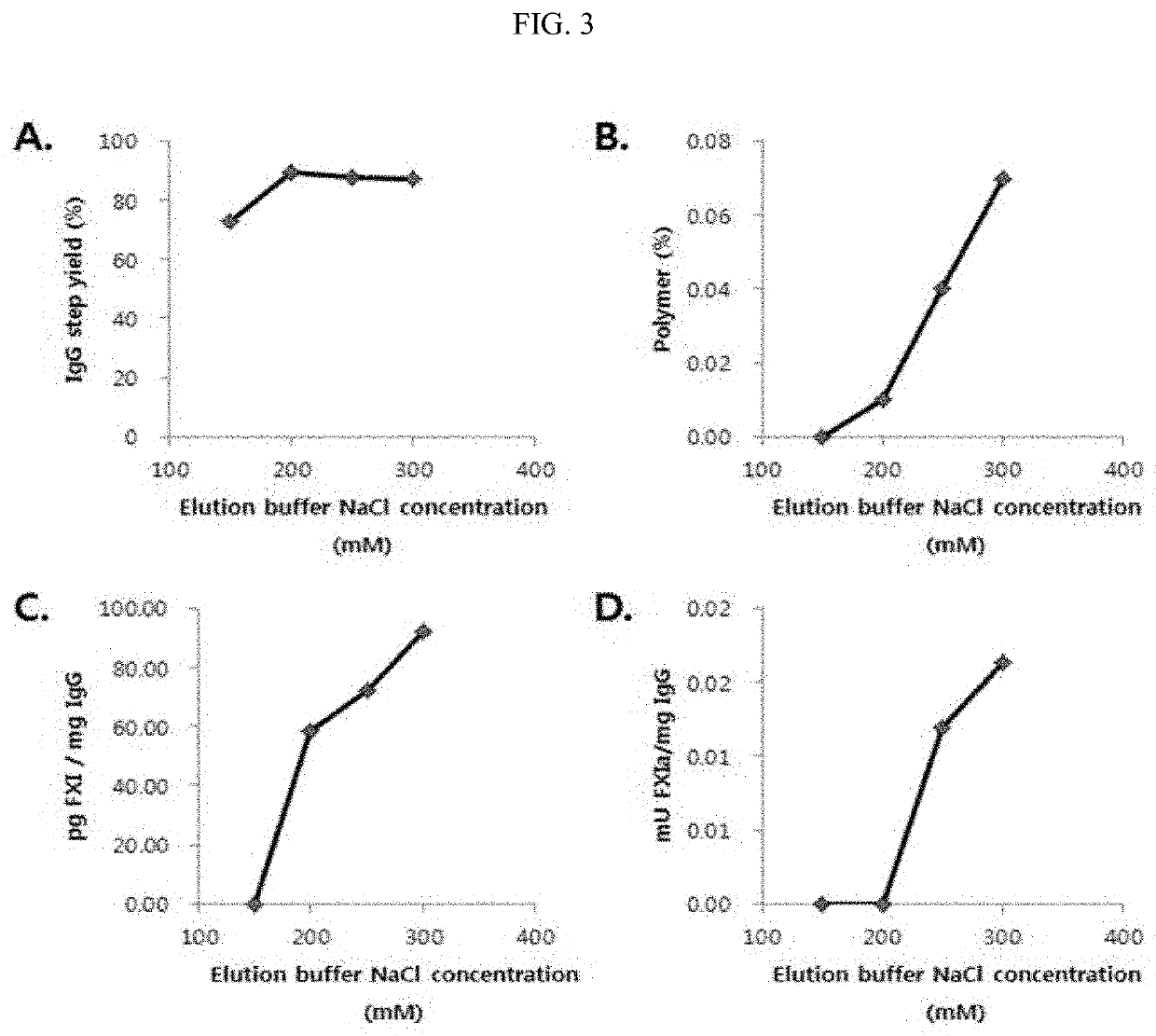
[0079]1.1 Purification Column Sequence Process Study
[0080]1.1.1. Cation-Exchange Chromatography (CEX) Experimental Conditions after Anion-Exchange Chromatography (AEX)[0081]Fraction I+II+III obtained from human plasma was used and prepared according to a conventional Cohn plasma fractionation method.[0082]Fraction I+II+III Paste Dissolution
[0083]Fraction I+II+III paste was stored at a temperature of −30° C. or less and dissolved for 15-21 hours at 2° C. to 8° C. before use.
[0084]The dissolved fraction I+II+III paste was dissolved for 30 minutes or longer by adding water for injection, and then adjusted to pH 4.0 using 1M acetic acid or 0.5 N NaOH.
[0085]Caprylic Acid Precipitation
[0086]A 1M caprylic acid solution was added so that the caprylic acid concentration of the solution was 19 mM to 21 mM.
[0087]Filtration
[0088]The precipitate solution was filtered using 2-μm and 18-μm filter membranes at a pressure of 2 bar to 3 bar.
[0089]To recover the residual IgG from a filt
example 2
[0160]1.1. Study for Process of Sequential Chromatography
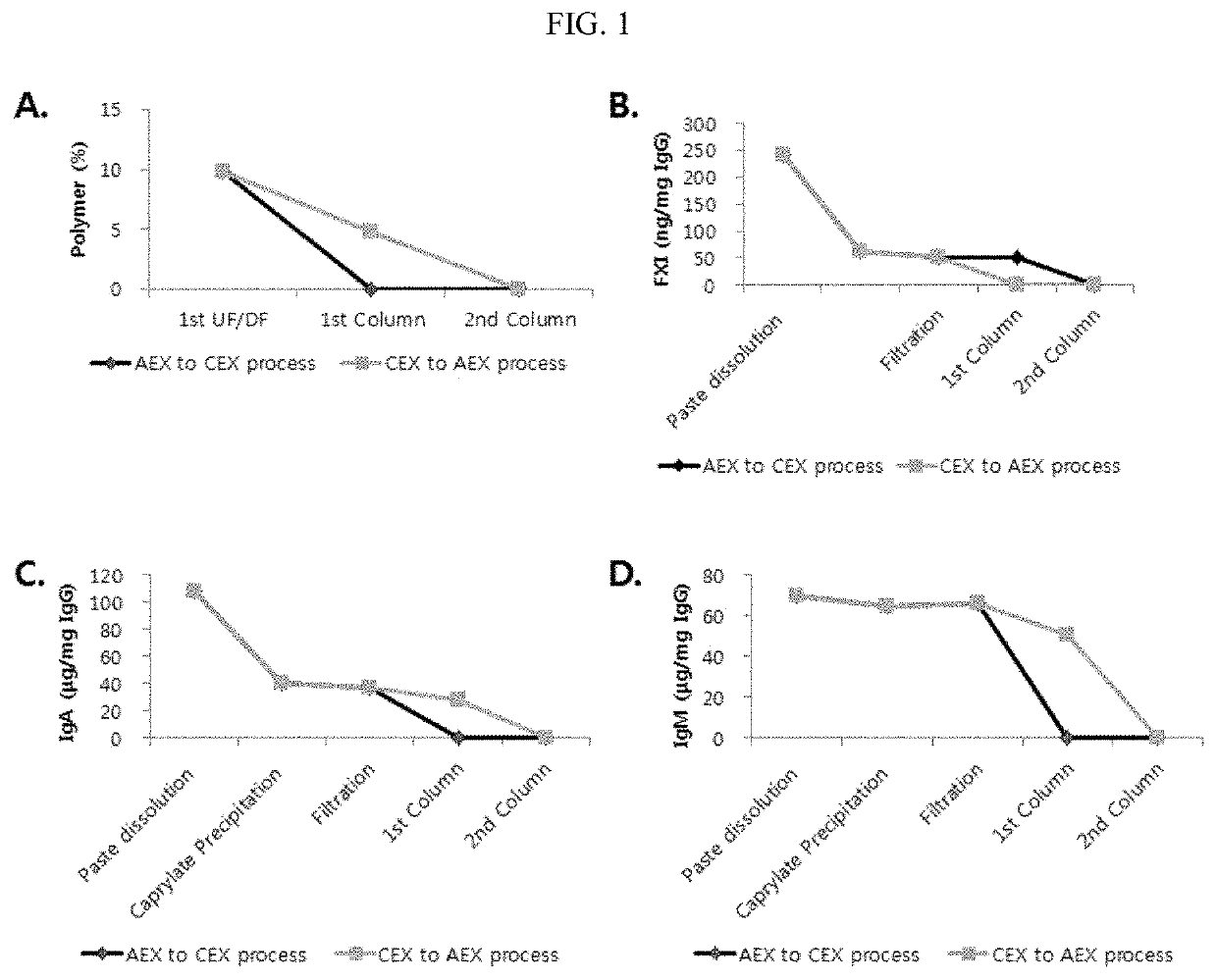
[0161]In the case of third-party processes for purifying plasma-derived immunoglobulins, many types of ion-exchange chromatography (IEX) are used to remove impurities. In the case of a process using both AEX and CEX, CEX is often used as a capture (1st column) step and AEX is used as a polishing (2nd column) step, and in this case, for the subsequent process, de-salting of the CEX process-completed solution is added, which complicates the overall process and reduces yield.
[0162]In contrast, in the case where AEX is used as a capture (1st column), the process is simplified, but it is required to develop a process suitable for completely removing major impurities.
[0163]As a result of conducting a comparison, in the case where AEX was used as the 1st column and CEX was used as the 2nd column, the number of overall purification processes was reduced and total yield was increased, and it was confirmed that the case satisfied acceptanc
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap