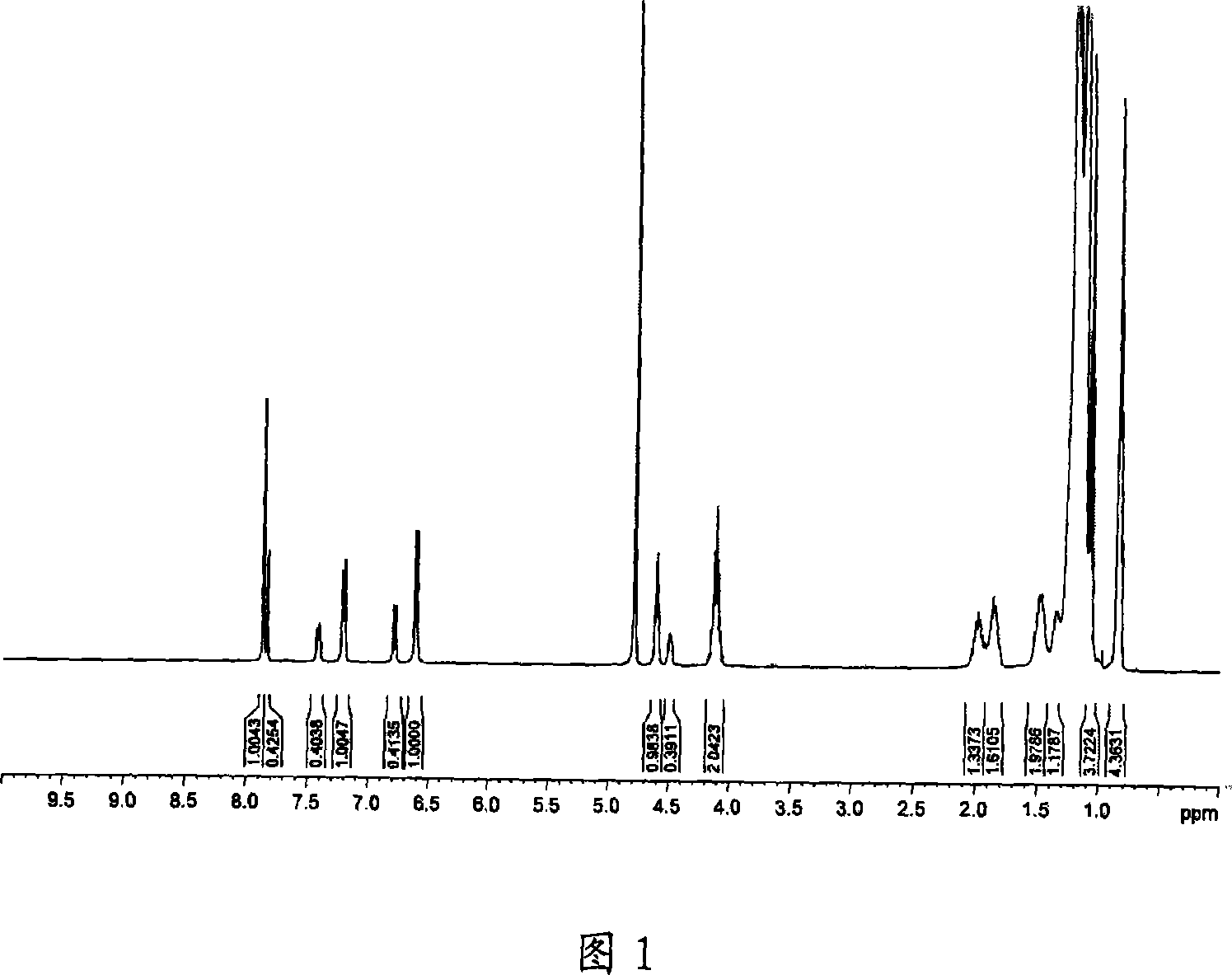
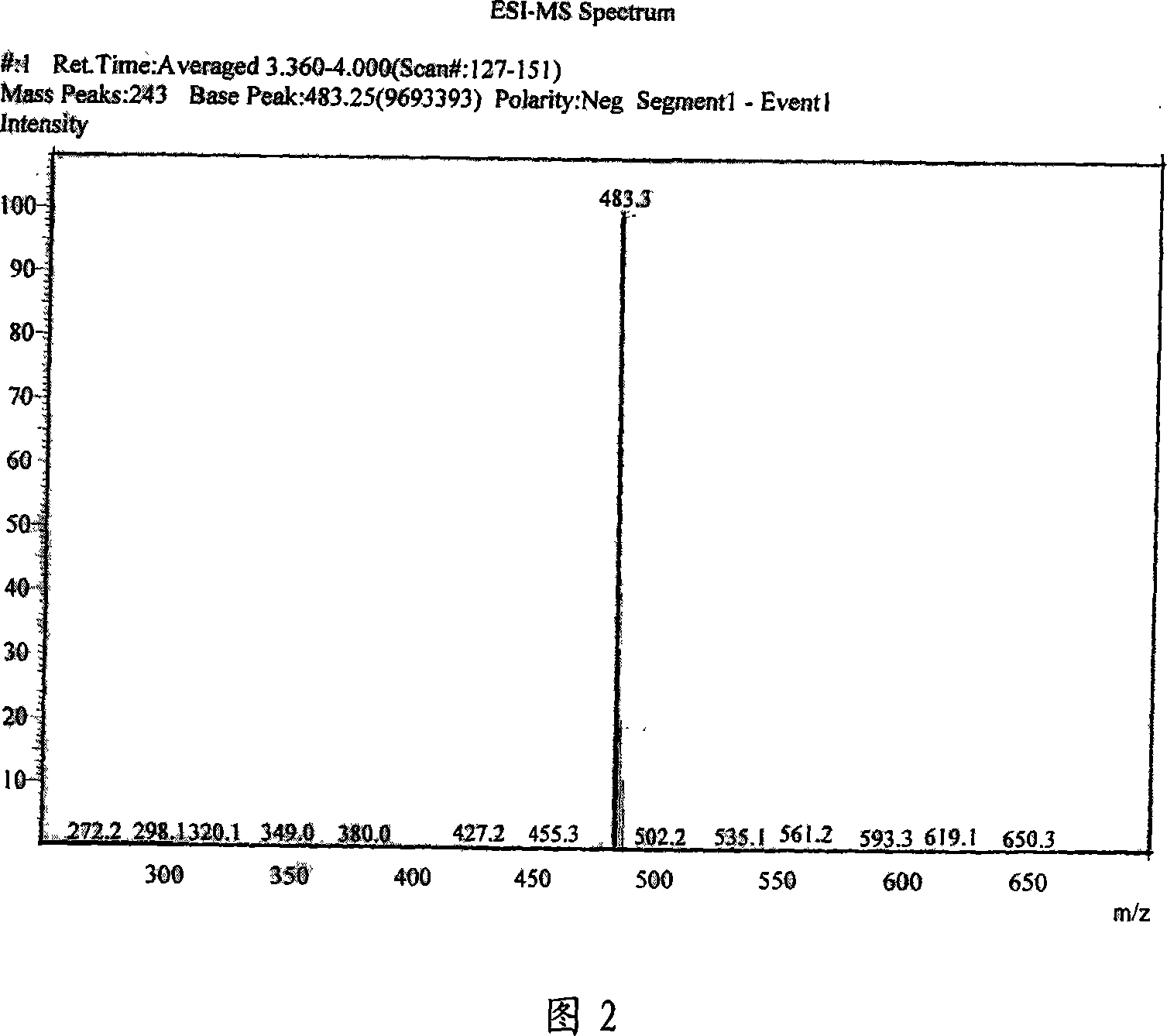
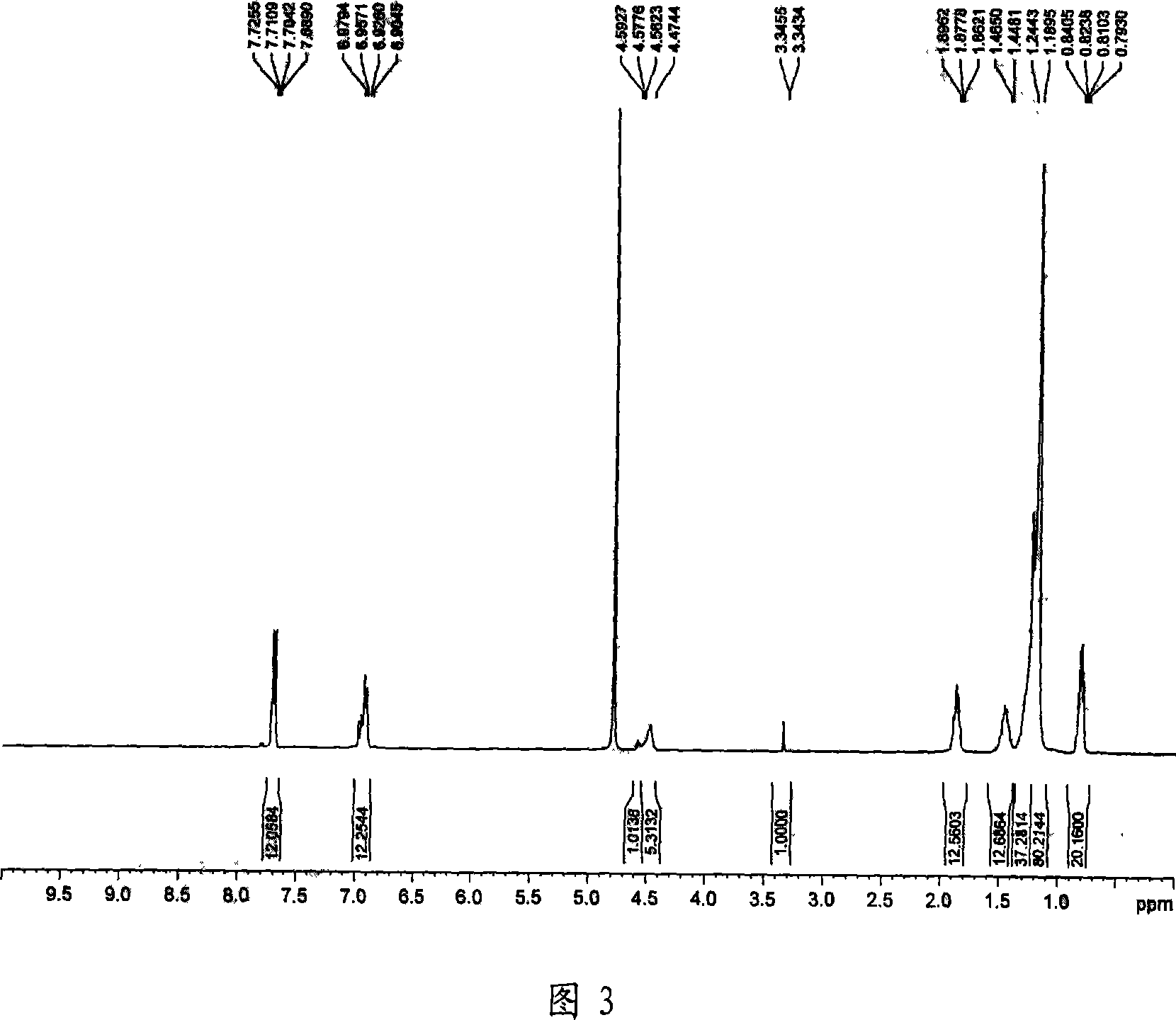
Asymmetric Gemini surface activator, its production and use
A surfactant and asymmetric technology, applied in chemical instruments and methods, transportation and packaging, dissolution, etc., can solve problems such as high production cost, difficult synthesis of asymmetric Gemini surfactants, and obstacles to popularization and application of Gemini surfactants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067]The first step: get α-bromotetradecanoic acid ethyl ester, p-tert-butylphenol and potassium carbonate in a three-necked flask, the mol ratio of α-bromotetradecanoic acid ethyl ester: p-tert-butylphenol: potassium carbonate 1.1:1:1.5; dissolve the above mixture in a mixed solvent of N,N'-dimethylformamide:toluene with a volume ratio of 3:1, heat up to boiling under stirring, reflux to separate water, and wait for no water to come out Then stop heating, filter the reaction solution after cooling, transfer the filtrate to a separatory funnel, add water to separate the liquid, and dry the separated organic phase with anhydrous magnesium sulfate. After filtering, evaporate the organic solvent and carry out vacuum distillation to obtain α- Ethyl (p-tert-butyl)phenoxytetradecanoate.
[0068] The second step: take α-(p-tert-butyl)phenoxytetradecanoic acid ethyl ester in a three-necked flask filled with 1,2-dichloroethane, and add chlorosulfonic acid dropwise to the three-necked fla
Embodiment 2
[0070] The first step: get α-bromotetradecanoic acid ethyl ester, phenol and salt of wormwood in the reactor, the mol ratio of α-bromoteristate ethyl ester: phenol: potassium carbonate is 1.1: 1: 1.5; Dissolve the mixture in a mixed solvent of N,N'-dimethylformamide:benzene with a volume ratio of 3:1, heat up to boiling with stirring, reflux to separate water, stop heating after no water is separated, and filter the reaction solution after cooling , the filtrate was transferred to a separatory funnel, water was added for liquid separation, the separated organic phase was dried with anhydrous calcium chloride, the organic solvent was evaporated after filtration, and the vacuum distillation was carried out to obtain ethyl α-phenoxytetradecanoate .
[0071] The second step: take α-phenoxytetradecanoic acid ethyl ester in a three-necked flask filled with 1,2-dichloroethane, add chlorosulfonic acid dropwise to the three-necked flask under stirring, α-phenoxy The ethyl carboxylate:chl
Embodiment 3
[0073] The first step: get α-bromotetradecanoic acid ethyl ester, p-tert-butylphenol and potassium carbonate in a three-necked flask, the mol ratio of α-bromotetradecanoic acid ethyl ester: p-tert-butylphenol: potassium carbonate 1.1:1:1.5; dissolve the above mixture in a mixed solvent of N,N'-dimethylformamide:toluene with a volume ratio of 3:1, heat up to boiling under stirring, reflux to separate water, and wait for no water to come out Then stop heating, filter the reaction solution after cooling, transfer the filtrate to a separatory funnel, add water to separate the liquid, and dry the separated organic phase with anhydrous magnesium sulfate. After filtering, evaporate the organic solvent and carry out vacuum distillation to obtain α- Ethyl (p-tert-butyl)phenoxytetradecanoate.
[0074] The second step: take α-(p-tert-butyl)phenoxytetradecanoic acid ethyl ester and dissolve it in ethanol, add an aqueous solution of sodium hydroxide to it, and use α-(p-tert-butyl)phenoxytetra
PUM
| Property | Measurement | Unit |
|---|---|---|
| Salinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap