Method for amplifying and activating lymphocyte cells by CD 8 alpha-interleukin 21 fragment-CD137 complex
A-CD137, lymphocyte technology, applied in the field of immunology, can solve the problems such as the need to improve the amplification and activation effect, the long fragment, and the difficulty in obtaining a large number of LAK cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
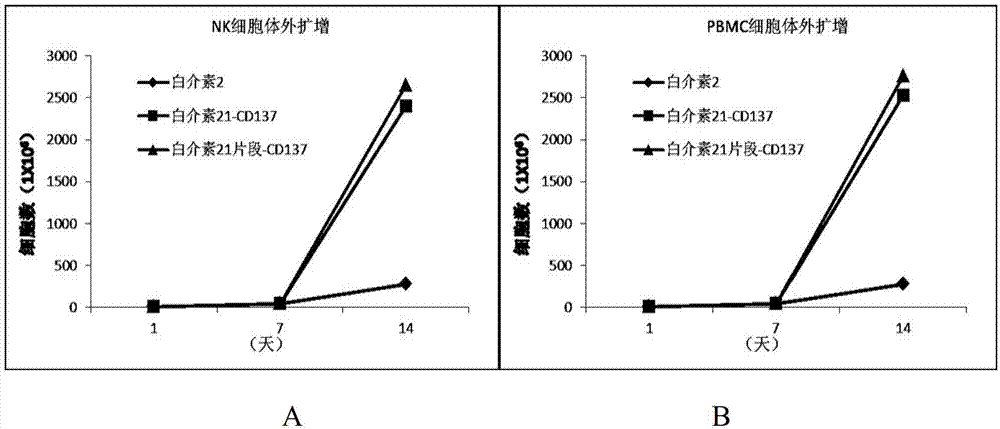
[0045] Transmembrane interleukin 21 fragment-CD137 complex expands LAK cells in vitro.
[0046] NK cells from healthy donors were cultured in RPMI1640 supplemented with 10% human serum. K562 cells expressing transmembrane interleukin-21-CD137 complex (control) and K562 cells expressing transmembrane interleukin-21 fragment-CD137 complex (irradiated, 100Gy) and low-dose interleukin-2 were added to the culture medium and cultured for 7 days . Centrifuge after 7 days, resuspend with an equal amount of culture medium, add irradiated K562 cells, and culture for another 7 days ( figure 1 ). High-dose interleukin-2 (200 units / ml) was also used as a control group. Such as figure 2 As shown in A, the number of LAK cells was significantly increased under the joint action of transmembrane interleukin-21 fragment-CD137 complex and low-dose interleukin-2. An increase in cell number can be measured by the insertion of thymidine. Cells were cultured for 12 hours after thymidine inserti
Embodiment 2
[0048] Transmembrane interleukin 21 fragment-CD137 complex expands unpurified peripheral blood lymphocytes
[0049] The transmembrane interleukin 21 fragment-CD137 complex can expand not only purified NK cells, but also unpurified lymphocytes. PBM from healthy donors were cultured in RPMI1640 supplemented with 10% human serum. K562 cells expressing transmembrane interleukin-21-CD137 complex (control) and K562 cells expressing transmembrane interleukin-21 fragment-CD137 complex (irradiated, 100Gy) and low-dose interleukin-2 were added to the culture medium and co-cultured for 7 sky. Centrifuged after 7 days, resuspended with an equal amount of culture medium, added K562 cells irradiated by 100Gy, and cultured for another 7 days ( figure 1 ). High-dose interleukin-2 (200 units / ml) was also used as a control group. Such as figure 2 As shown in B, the number of LAK cells was significantly increased under the joint action of transmembrane interleukin-21 fragment-CD137 complex
Embodiment 3
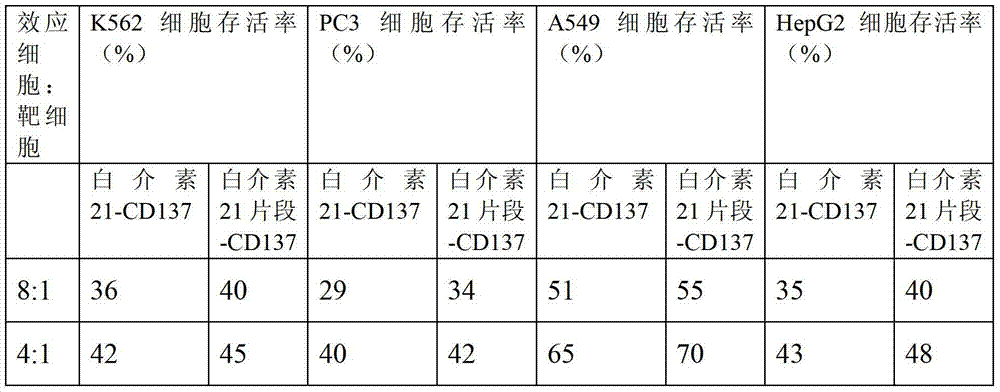
[0052] Detection of Telomerase Activity and STAT3 Phosphorylation in LAK Cells
[0053] The amplified cells were disrupted by repeated freezing and thawing with liquid nitrogen, centrifuged at 10,000 g, and the supernatant was taken, and the telomerase activity of the amplified LAK cells was detected by the TRAP method. 50 μl TRAP system contains 20 mmol / L Tris-HCl (pH8.3), 1.5 mmol / L MgCl, 63 mmol / L, KCl, 0.005% Tween-20, 1 mmol / L EDTA, 50 Millimolar / L dNTP, 0.1 μg tS, 1 μg T4, 0.1 mg / ml bovine serum albumin, 1-2 μl CHAPS, cell extract (including 6 μg) protein, 0.2-0.4 μl [α- 32 P]dGTP. Reaction conditions: 10 minutes at 23°C, 3 seconds at 94°C, add Taq enzyme, 30 seconds at 94°C, 30 seconds at 50°C, 1.5 minutes at 72°C, 27 cycles of amplification, take 25 μl of product for 15% PAGE gel electrophoresis. Autoradiography on X-ray film after PAGE gel electrophoresis for 8 hours to more than two days. Compared with before amplification, the high dose of interleukin 2
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap