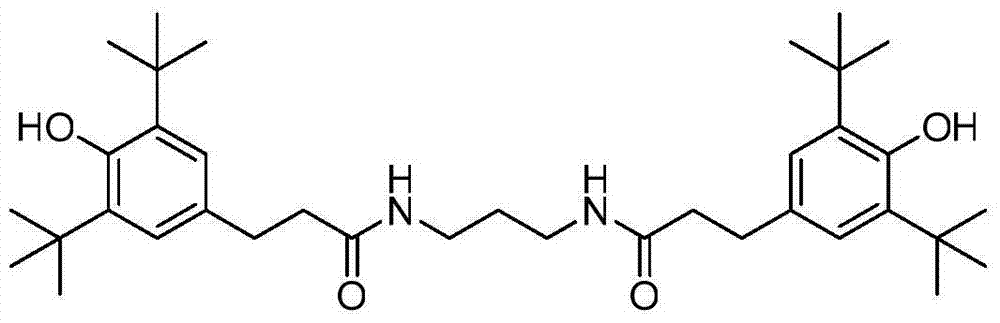
Preparation method of hindered phenol antioxidant 1019
A technology of hindered phenols and antioxidants, which is applied in the field of preparation of polymer antioxidants, can solve the problems of low actual yield, low reaction yield, low yield and the like, and achieves mild reaction conditions, simple operation and easy operation. The effect of separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Dissolve 100g of 3,5-acyl chloride in 500mL of n-hexane, and gradually add the configured n-hexane solution of 3,5-acyl chloride into a solution containing 12.0g of propylenediamine, 20.0g of sodium hydroxide and 200mL of water under stirring In the mixed solution, the temperature of the reaction system was controlled to be 40° C. during the addition, and then the reaction was continued at 40° C. for 2 hours to the end of the reaction. After the reaction was completed, the temperature was raised to 60°C, the lower aqueous phase was separated, and the organic phase was washed with water for 2 to 3 times until the pH of the washing water was 6 to 7, then cooled to crystallize, filtered, and then dried to obtain the target product N,N'-bis (3-(3,5-Di-tert-butyl-4-hydroxyphenyl)propionyl)propionyl)diamine 94.3g, yield 97.9% (calculated as propylenediamine), HPLC content 99.2%.
Embodiment 2
[0020] Dissolve 100g of 3,5-acyl chloride in 400mL of cyclohexane, and gradually add the configured cyclohexane solution of 3,5-acyl chloride to 10.0g of propylenediamine, 17.8g of sodium carbonate and 100mL of water under stirring In the mixed solution, the temperature of the reaction system was controlled to be 50° C. during the addition, and then the reaction was continued at 50° C. for 1 hour to the end of the reaction. After the reaction was completed, the temperature was raised to 60°C, the lower aqueous phase was separated, and the organic phase was washed with water for 2 to 3 times until the pH of the washing water was 6 to 7, then cooled to crystallize, filtered, and then dried to obtain the target product N,N'-bis (3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionyl)propionyl)diamine 77.8g, yield 96.9% (calculated as propylenediamine), HPLC content 98.9%.
Embodiment 3
[0022] Dissolve 100g of 3,5-acyl chloride in 300mL of methylcyclohexane, and gradually add the prepared 3,5-acyl chloride in methylcyclohexane to the solution containing 12.0g of propylenediamine, 48.6g of potassium carbonate and 500mL of water, the temperature of the reaction system was controlled to be 40°C when adding, and then the reaction was continued at 40°C for 2 hours to the end of the reaction. After the reaction was completed, the temperature was raised to 60°C, the lower aqueous phase was separated, and the organic phase was washed with water for 2 to 3 times until the pH of the washing water was 6 to 7, then cooled to crystallize, filtered, and then dried to obtain the target product N,N'-bis (3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionyl)propionyl)diamine 95.0g, yield 98.7% (calculated as propylenediamine), HPLC content 99.0%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap