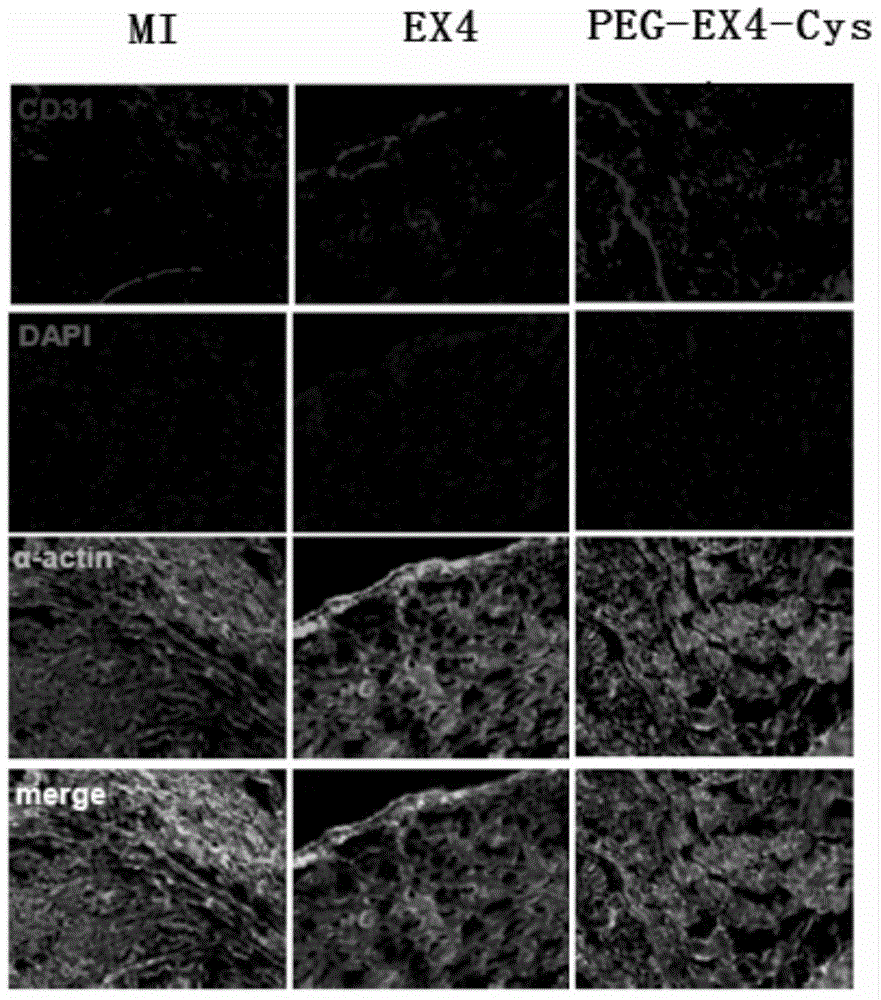
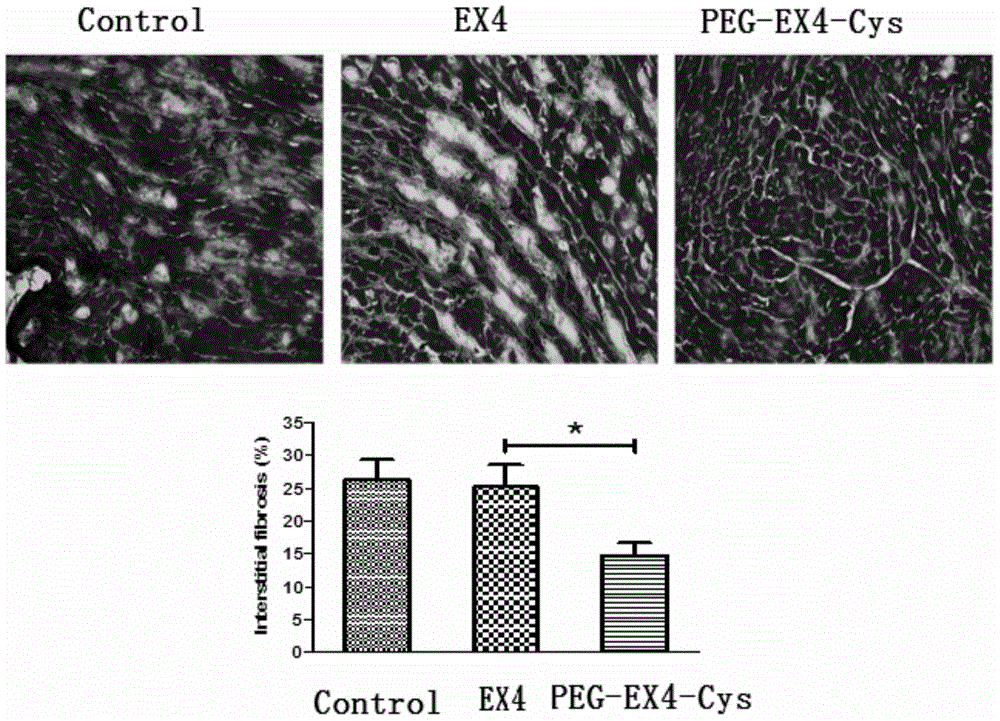
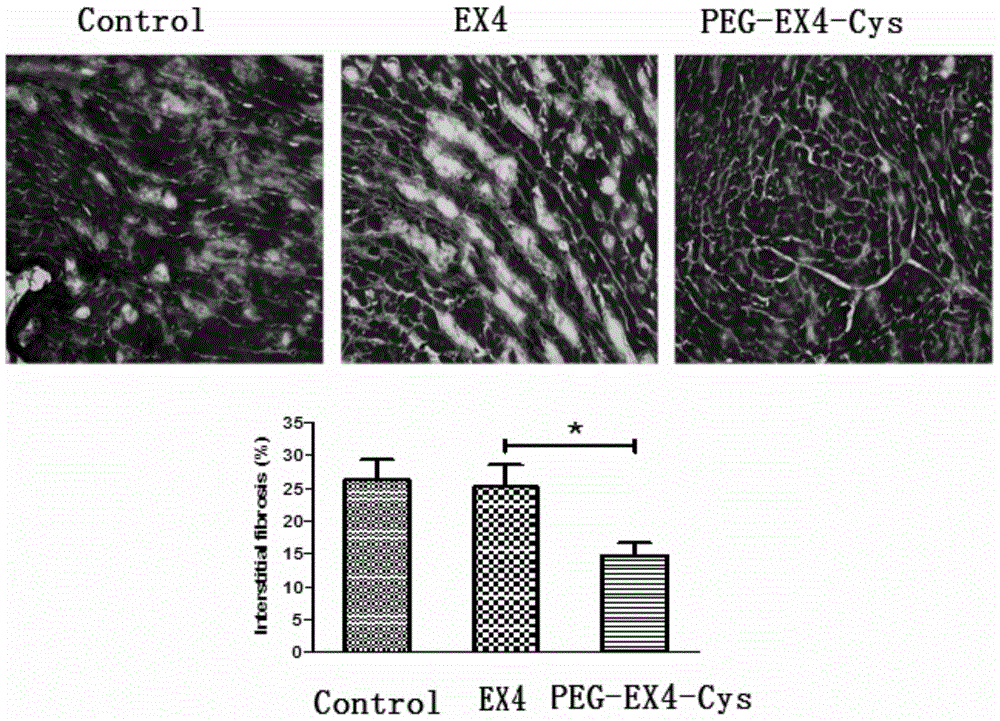
Polyethylene glycol-modified exendin analog, and preparation method and application thereof
A technology of polyethylene glycol and mpeg-l-s-cysex4, applied in the field of biomedicine, can solve problems such as poor specificity and prolongation of half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Example 1. Preparation of linear polyethylene glycol modified Ex4-Cys (C40-PEG-Ex4-Cys)
[0100] A polypeptide (Ex4-Cys) with the following sequence was synthesized using standard polypeptide solid-phase synthesis technology:
[0101] His-Gly-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Leu-Ser-Lys-Gln-Met-Glu-Glu-Glu-Ala-Val-Arg-Leu-Phe-Ile-Glu-Trp- Leu-Lys-Asn-Gly-Gly-Pro-Ser-Ser-Gly-Ala-Pro-Pro-Pro-Pro-Ser-Cys; (Seq ID No.1)
[0102]3, 5, 10, 20 and 50 kDa polyethylene glycols with maleine groups (Nektar, mPEG-pro-pionalaldehyde, mPEG-ALD, 2 kDa, 0.95 mg / ml in 50 mM sodium acetate, pH 5.5) Add respectively 0.5mL Exendin-4 (1 mg / ml in 50mM sodium acetate, pH 5.5), and then add 20mM NaC-NBH3 as reducing agent. The molar ratio of mPEG-ALD to Ex4-Cys is 1:1-2. mPEG-ALD and Ex4-Cys were reacted at 4°C for 2 hours under dark conditions. The reaction was terminated with 0.1% aqueous trifluoroacetic acid (TFA) to obtain PEG-modified Ex4-Cys, named as C40-PEG-Ex4-Cys.
Embodiment 2
[0103] Example 2. Preparation of branched polyethylene glycol modified EX4-Cys (C40-tPEG-Ex4-Cys)
[0104] Add 0.5 mL Ex4-Cys (1 mg / ml in 50 mM sodium acetate, pH 5.5), then 20 mM NaC-NBH 3 as a reducing agent. The molar ratio of mPEG-ALD to Ex4-Cys is 1:1-2. mPEG-ALD and Ex4-Cys were reacted at 4°C for 2 hours under dark conditions. The reaction was terminated with 0.1% aqueous trifluoroacetic acid (TFA) to obtain branched PEG-modified Ex4-Cys with different molecular weights, named C40-tPEG-Ex4-Cys. Purified C40-tPEG-Ex4-Cys as figure 1 Shown, its retention time is 18.5 minutes.
Embodiment 3
[0105] Example 3. Analysis of the influence of PEG modification on the physiological activity image of Ex4-Cys
[0106] To detect Exendin-4, PEG-modified Ex4-Cys of different molecular weights reacted with the GLP-1 receptor at a density of 2.5×10 5 The insulin-secreting cells INS-1 were seeded on a 12-well plate, 10 per well 5 cells, and then cultured for 2 days to allow them to stably attach to the bottom of the culture plate. After the cells adhered to the wall, labeled with 125 The buffer of I-Exendin-4 (Exendin-4 derivative extending from amino acid residue 9 and amino acid residue 39) was used to replace the cell culture medium, and a certain amount of buffer was added to form a final concentration of 30 μM. Thereafter, a certain amount of natural Exendin-4 and PEG-modified Ex4-Cys of different molecular weights were added to form a final concentration of 0.001-1000 nM, and incubated at room temperature for 2 hours to enable it to competitively bind to the receptor. Wash
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap