Preparation method of 3,5-di-tert-butyl-4-hydroxybenzyl alcohol
A technology of di-tert-butyl and hydroxybenzyl alcohol, which is applied in the preparation of organic compounds, chemical instruments and methods, and preparation of carbonyl compounds by condensation, etc., can solve the problems of complex separation and purification process, disadvantage, and influence on the development and application of downstream products, etc. To achieve the effect of high reaction yield and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
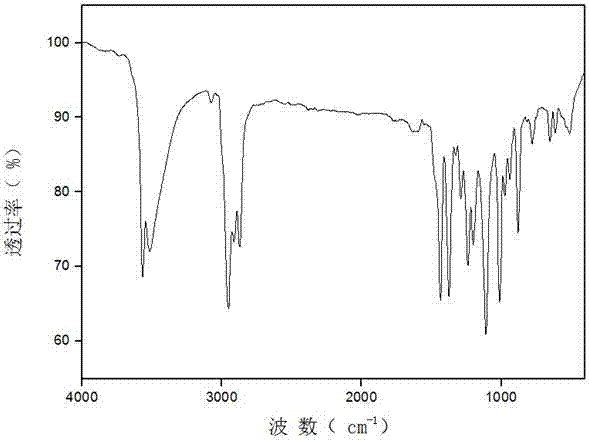
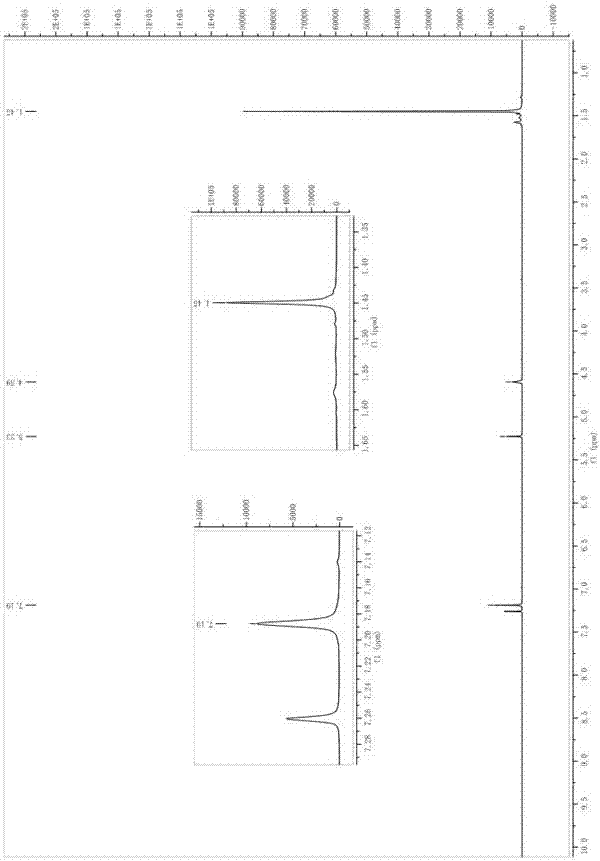
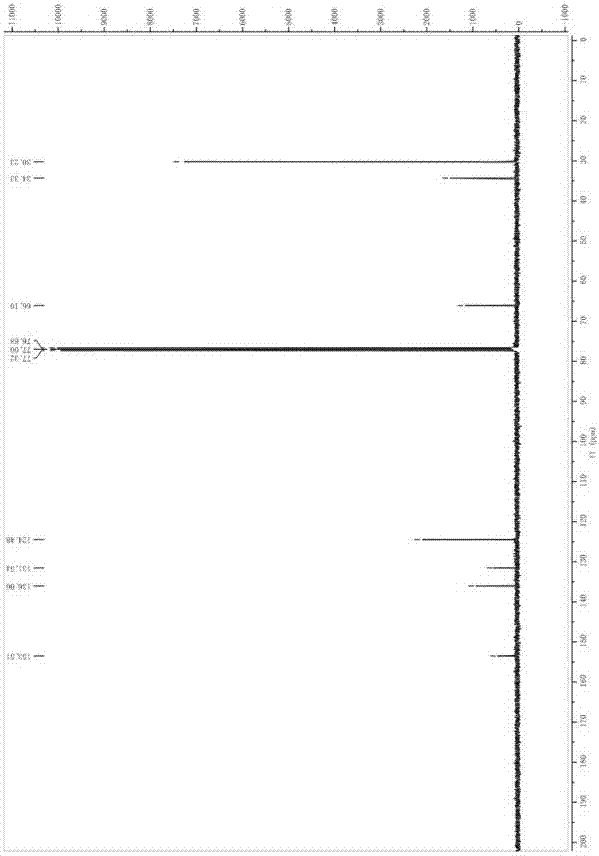
[0018] Synthesis of 3,5-di-tert-butyl-4-hydroxybenzaldehyde
[0019] Take 2.42mL POCl 3 (0.026mol) was added to 2.01mL DMF (0.026mol) at 0°C, stirred until a white solid was produced, and dissolved in 20mL 1,2-dichloroethane to obtain solution A. Dissolve 4.12g of 2,6-di-tert-butylphenol (0.02mol) in 20mL of 1,2-dichloroethane to obtain solution B. Add solution B dropwise to solution A at room temperature, reflux at 70°C for 2 hours, add saturated Na 2 CO 3 The solution was neutralized until no gas was produced, then refluxed at 80°C for 40 minutes, separated, the organic phase was washed with water, and Na 2 CO 3 Wash with saturated solution and dry. The organic phase was distilled off under reduced pressure to obtain 4.48 g of light brown flaky solid with a yield of 96% and a melting point of 185-189°C (literature value 186-190°C);
[0020] Synthesis of 3,5-di-tert-butyl-4-hydroxybenzyl alcohol
[0021] Take 4 g of 3,5-di-tert-butyl-4-hydroxybenzaldehyde (0.017 mol) prep
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap