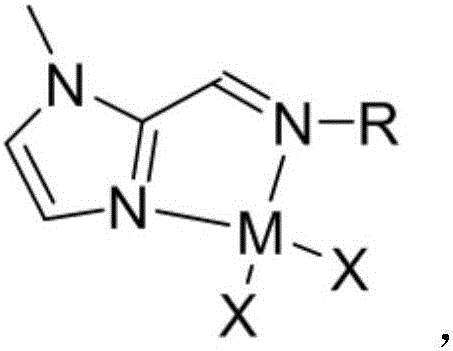
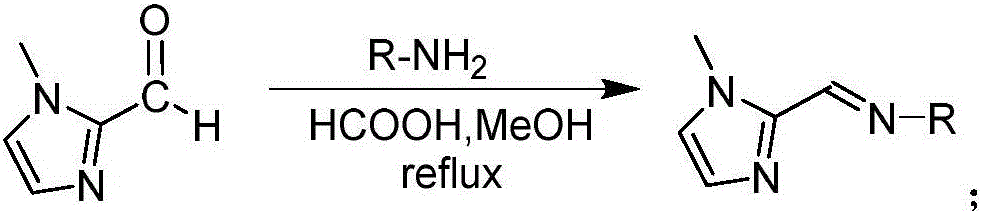Imidazolamide ligand and nickel complex thereof as well as application of nickel complex to polyisoprene synthesis
A technology of imidazolium nickel and imidazolium, which is applied in the field of new compounds and organic synthesis, to achieve the effect of reducing the requirements of reaction conditions, improving controllable polymerization, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The preparation of embodiment 1 imidazole imine ligand
[0055] All ligands described here are obtained by condensation reactions of substituted aldehydes or ketones with primary amines. Complex amines need to be synthesized in advance. Many commercially available amines and substituted aldehydes and ketones can be subjected to one-step synthesis of ligands (imine condensation).
[0056] (1) Synthesis of imidazole imine ligand compound a
[0057]
[0058] Under the protection of Ar, 10 mL of methanol solution dissolved in 1-methyl-imidazole-2-carbaldehyde (275.3 mg, 2.5 mmol, 1 eq.), tert-octylamine (0.48 mL, 3 mmol, 1.2 eq.), formic acid (0.1 mL), reflux reaction at 80°C, TLC tracking detection until 1-methyl-imidazole-2-carbaldehyde raw material disappeared. The solvent was spin-dried, and the unreacted raw material tert-octylamine was distilled off under reduced pressure, and the remaining light red liquid was the target product with a yield of 66%.
[0059] (2)
Embodiment 2
[0062] The synthesis of embodiment 2 imidazolium nickel complexes
[0063] (1) Synthesis of imidazolium nickel complex c
[0064]
[0065] Inside the glove box, add anhydrous NiCl to a 10 mL Schlenk tube 2 (73.9mg, 0.57mmol, 1eq.), the imidazolium imine ligand compound a (72.3mg, 0.57mmol, 1eq.) prepared in Example 1 was added to 5mL of anhydrous DCM, and stirred at room temperature for 4 days. After the reaction is over, dissolve the precipitated solid with 10 mL×2 anhydrous DCM and filter, collect the DCM phase to obtain an orange liquid, drain the remaining DCM, wash twice with 20 mL of anhydrous n-hexane, remove the supernatant and The residue was dried to give a brown solid in 62% yield.
[0066] (2) Synthesis of imidazolium nickel complex d
[0067]
[0068] In the glove box, add anhydrous NiBr to a 10 mL Schlenk tube 2 (126.7mg, 0.58mmol, 1eq.), the imidazolium imine ligand compound a (75.4mg, 0.58mmol, 1eq.) prepared in Example 1 was added to 5mL of anhydrous TH
Embodiment 3
[0078] Take a 25mL Schlenk bottle, add cocatalyst MAO (4mmol, 500eq.), toluene 5mL successively therein, complex c (8μmol, 1eq.) is dissolved in 1mL dichloromethane, this mixed solution is stirred at room temperature 2min, then Isoprene monomer (20mmol, 2500eq.) was added, and after polymerization at 25°C for 2h, 1M methanolic hydrochloric acid solution was added to terminate the reaction. Pour the viscous polymer solution into 50 mL of ethanol and allow the polymer to settle out. The polymer after the supernatant was discarded was washed with distilled water and ethanol in sequence, and the obtained polymer was dried in a vacuum drying oven at 40° C. to a constant weight to obtain the product polyisoprene with a yield of 80%. The reaction selectivity was 89.7% trans-1,4 and 10.3% 3,4-polyisoprene.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap