Umbilical cord mesenchymal stem cells combined with medicine for treating hyperglycemia and diabetic nephropathy
A technology of diabetic nephropathy and mesenchymal stem cells, which is applied in the field of preparation of drugs for the treatment of hyperglycemia and diabetic nephropathy, can solve problems such as liver damage, edema and weight of patients, and achieve the effect of improving kidney function and controlling blood sugar levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1 Human umbilical cord mesenchymal stem cell culture and passage method
[0048] After primary culture, huMSCs (i.e. human umbilical cord mesenchymal stem cells) were placed in DMEM / F12 medium containing 10% FBS, 100U / ml penicillin and streptomycin; then, cultured at 37°C, 5% CO2 Routine culture and subculture in the box; the cells obtained from the subculture were digested with 0.25% trypsin, and the stably proliferating cells were collected.
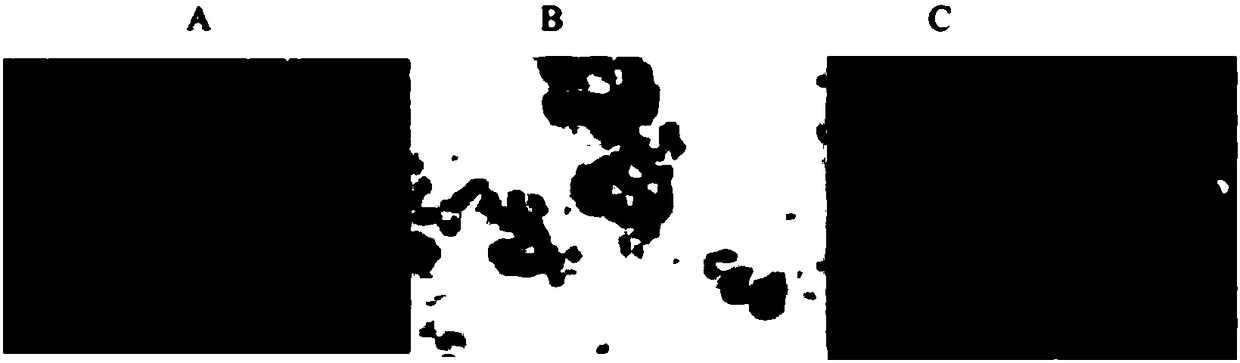
[0049] will be 10 6 The collected human umbilical cord mesenchymal stem cells were suspended in 100ml PBS buffer solution containing 1% bovine serum albumin, and positive antibodies (CD90, CD105, CD73) and negative antibodies (CD45, CD34, CD11b) were detected by flow cytometry , CD19 and HLA-DR) expression. see results figure 1 , showing that the cultured human umbilical cord mesenchymal stem cells meet the standard of stem cells.
Embodiment 2
[0050] Embodiment 2, the preparation of Chinese medicine extract
[0051] Each Chinese medicine extract is prepared according to the following method:
[0052] Take ginseng, salvia miltiorrhiza, and astragalus, slice, filter, dry and grind into powder, pass through a 80-120 mesh sieve to obtain fine powder, and set aside.
[0053] Crush schisandra and Panax notoginseng, put them in a container, immerse in 6-16 times the volume concentration of 50-80% ethanol solution for 1-10 hours, heat and reflux extraction 3 times, each time for 1-6 hours, filter, and combine the filtrates , ethanol was distilled off under reduced pressure and concentrated to a concentrated solution with a relative density of 1.10-1.13 at 60°C for later use.
[0054] Atractylodes macrocephala was decocted and extracted three times with 12 times, 10 times and 8 times the amount of water respectively, each time for 2 hours, the three water extracts were combined, concentrated under reduced pressure to 1g medici
experiment example 3
[0055] Experimental Example 3 Effects of Chinese Medicine Extracts on the Growth of Human Umbilical Cord Mesenchymal Stem Cells and Glomerular Cells
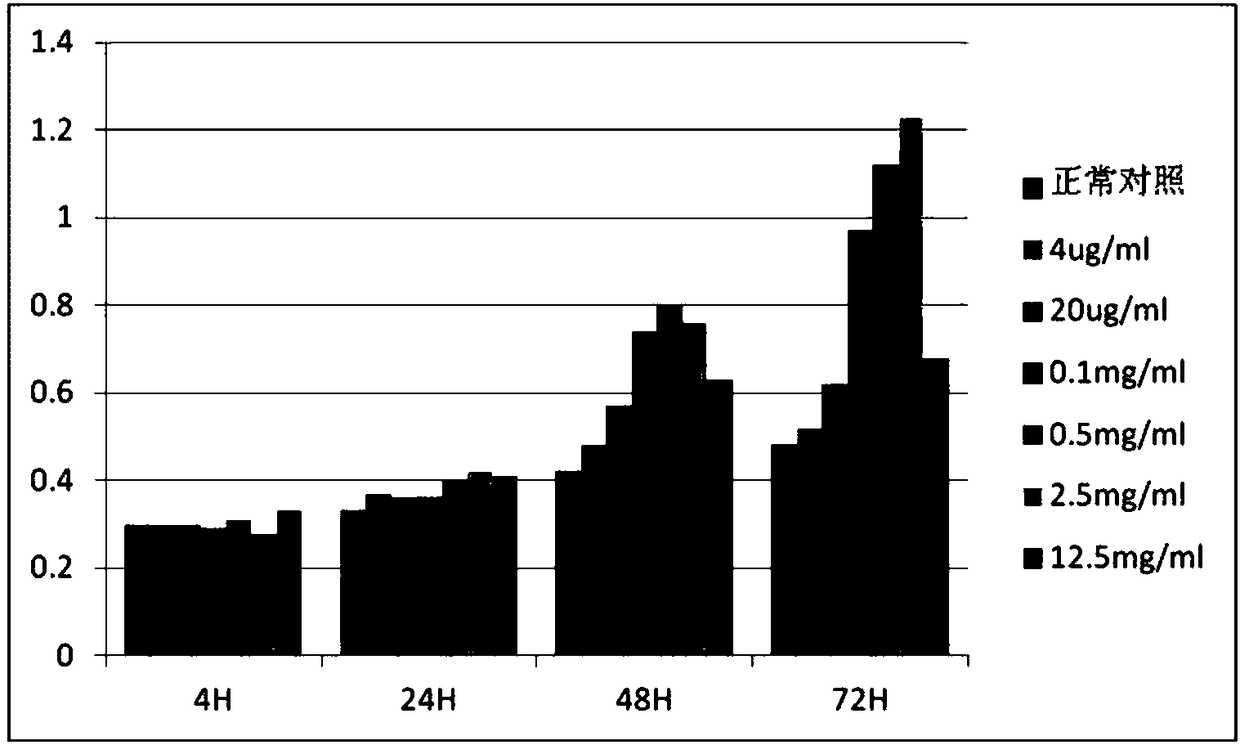
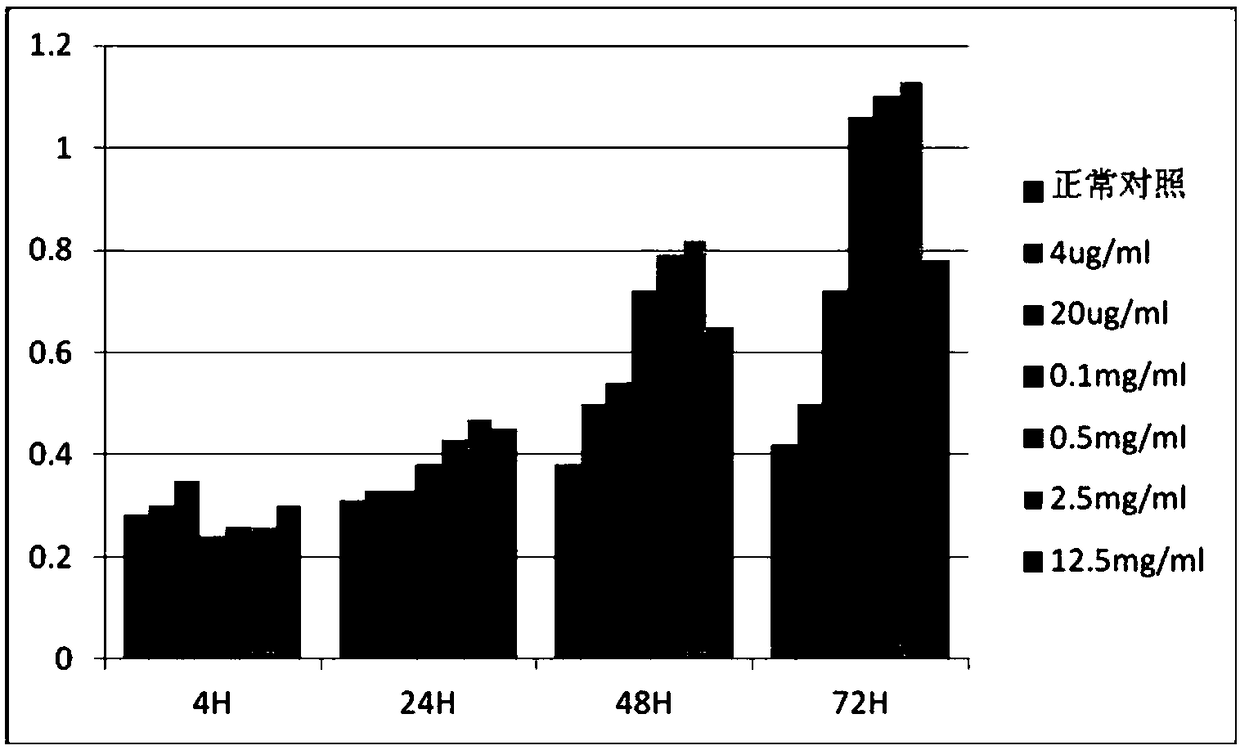
[0056] Select huMSCs (human umbilical cord mesenchymal stem cells) and HMC (glomerular cells) in good growth state to divide 3×10 3 Each well was inoculated in a 96-well plate. After the cells adhered to the wall, different concentrations of Chinese herbal extracts were added for co-cultivation. The control group did not add Chinese herbal extracts, and the zero wells did not contain cells. Only 100 μL of culture solution was added, and each group was parallelized. Make 5 duplicate wells, add 10 μL CCK8 solution to each group after culturing for 4h, 24h, 48h, and 72h, incubate at 37°C for 4h, select 490nm wavelength to measure the absorbance (OD) value of each well. Effective concentration measured according to cck-8 results. The test results are shown in Figure 2 and Figure 3.
[0057] The test results show that the traditional
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap