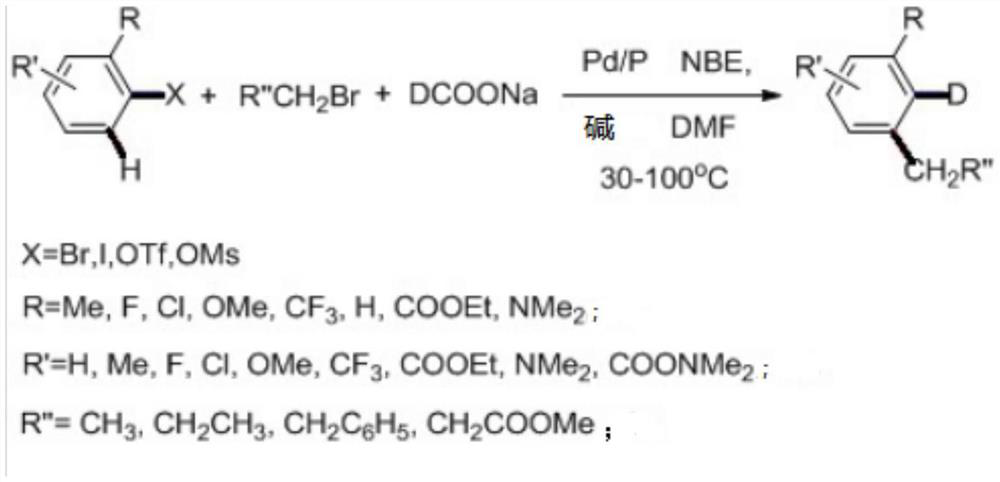
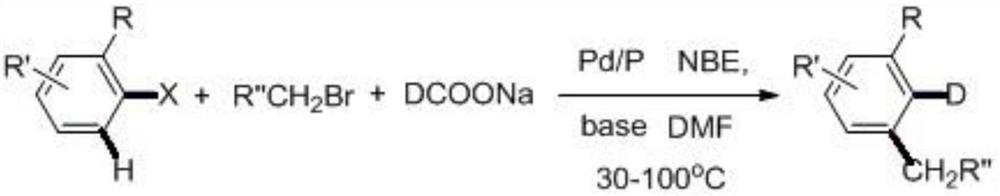
A kind of preparation method of deuterated aromatic compound
A technology for aromatic compounds and target compounds, which is applied in the field of preparation of deuterated aromatic compounds, can solve problems such as difficult application of complex deuterated compounds, and achieve the effects of avoiding multi-step synthesis methods, mild conditions, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
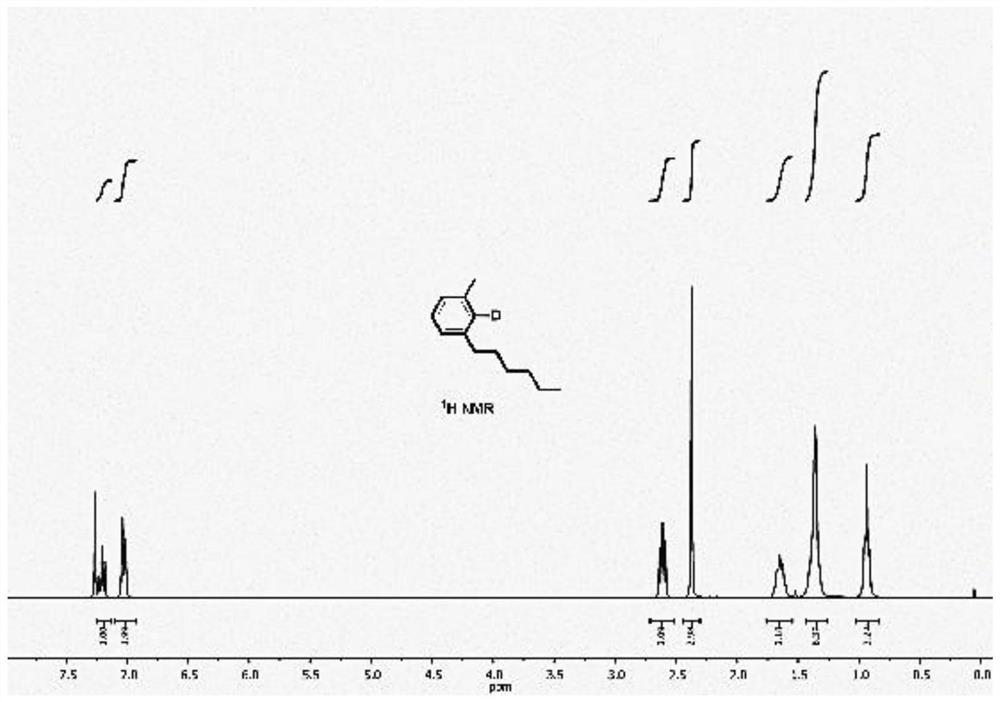
[0037] Embodiment 1: the preparation method of 2-deuterated-4-hexyltoluene
[0038]Palladium acetate (44.9 mg, 0.2 mmol), triphenylphosphine (52.4 mg, 0.2 mmol), sodium deuteroformate (276 mg, 4 mmol), norbornene (188 mg, 2 mmol), phosphoric acid were successively added to the 25 mL eggplant flask. Potassium (2.544 g, 12 mmol), potassium acetate (392 mg, 4 mmol), 2-iodotoluene (436 mg, 2 mmol), bromohexane (1.32 g, 8 mmol) and dimethylformamide (10 ml). High-purity nitrogen was replaced three times, and after 16 hours of reaction at 50 °C, the mixed solution was extracted with dichloromethane and water, the organic phases were combined, the organic solvent was concentrated, and the product was further purified by column chromatography to obtain 2-deuterated-4-hexyltoluene. The yield was 58%. Deuteration rate>98%. 1HNMR: δ7.21(t,J=6.0Hz,1H),7.03(d,J=9.0Hz,2H),2.61(t,J=6.0Hz,2H),2.98(s,3H),1.67~1.63 (m, 2H), 1.43~1.28 (s, 6H), 0.94 (t, J=9.0Hz, 3H). 13C NMR: δ142.80, 137.58, 128
Embodiment 2
[0039] Example 2: Preparation method of 2-deuterated-4-(2-ethyl-1,3-dioxolane)toluene
[0040] Palladium acetate (44.9 mg, 0.2 mmol), triphenylphosphine (52.4 mg, 0.2 mmol), sodium deuteroformate (276 mg, 4 mmol), norbornene (188 mg, 2 mmol), phosphoric acid were successively added to the 25 mL eggplant flask. Potassium (2.544g, 12mmol), potassium acetate (392mg, 4mmol), 2-iodotoluene (436mg, 2mmol), 2-(2-bromoethyl)-1,3-dioxane (1.448g, 8mmol) and Dimethylformamide (10ml). High-purity nitrogen was replaced three times, and after 16 hours of reaction at 50°C, the mixed solution was extracted with dichloromethane and water, the organic phases were combined, the organic solvent was concentrated, and the product was further purified by column chromatography to obtain 2-deuterated-4-(2-ethyl acetate) -1,3-dioxolane) toluene, yield 74%. Deuteration rate>98%. 1H NMR: δ7.18 (t, J=7.5Hz, 1H), 7.01 (dd, J=7.4, 3.0Hz, 2H), 4.91 (t, J=4.7Hz, 1H), 3.94 (m, 4H), 2.73(dd, J=9.5, 6.9Hz, 2H)
Embodiment 3
[0041] Embodiment 3: the preparation method of 2-deuterated-3-methylbenzene butyric acid ethyl ester
[0042] Palladium acetate (44.9 mg, 0.2 mmol), triphenylphosphine (52.4 mg, 0.2 mmol), sodium deuteroformate (276 mg, 4 mmol), norbornene (188 mg, 2 mmol), phosphoric acid were successively added to the 25 mL eggplant flask. Potassium (2.544 g, 12 mmol), potassium acetate (392 mg, 4 mmol), 2-iodotoluene (436 mg, 2 mmol), ethyl bromobutyrate (1.56 g, 8 mmol) and dimethylformamide (10 ml). High-purity nitrogen was replaced three times, and after 16 hours of reaction at 50 °C, the mixed solution was extracted with dichloromethane and water, the organic phases were combined, the organic solvent was concentrated, and the product was further purified by column chromatography to obtain 2-deuterated-3-methylphenylbutyric acid. Ethyl ester, yield 65%. Deuteration rate>98%. 1H NMR: δ7.17 (t, J=7.5Hz, 1H), 6.99 (t, J=7.7Hz, 2H), 4.13 (q, J=7.1Hz, 2H), 2.61 (t, J=7.6Hz, 2H), 2.32(m, 5H),
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap