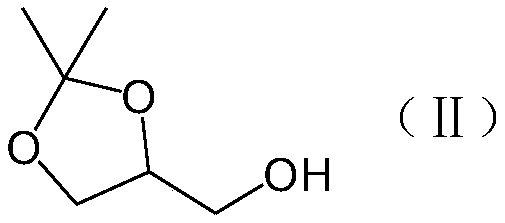
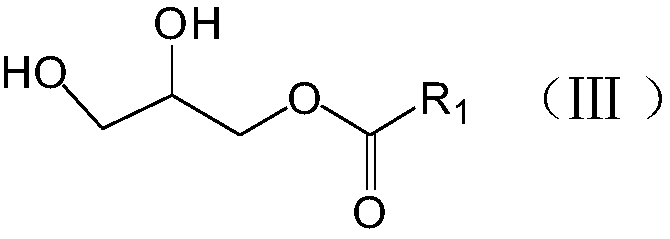
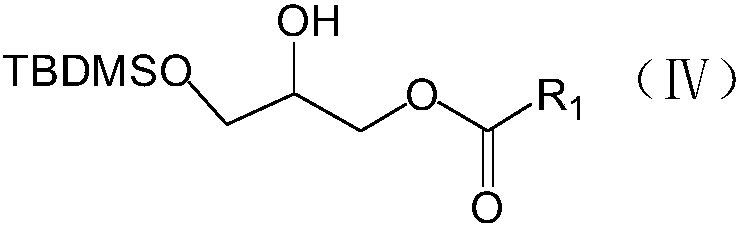
Preparation method of phosphatidylglycerol monosodium salt containing two different side chains
A technology of phosphatidylglycerol and monosodium salt, applied in chemical instruments and methods, edible phospholipid compositions, phosphorus organic compounds, etc., can solve problems such as application limitations, easy oxidation, poor stability, etc., to reduce production costs and simplify The effect of high process, yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Preparation of 1,1,5,6-isopropylidene-D-mannitol
[0042] Add 40 g of zinc chloride and 200 g of acetone into the reaction flask, stir at 30° C. for 30 min, then add 20 g of D-mannitol, and continue stirring for 10 h. After the reaction was completed, 100 mL of saturated sodium chloride solution was added, extracted with 100 mL*2 of dichloromethane, allowed to stand and separated, and the organic phases were combined. Add 200mL of 5% ammonia solution to the organic phase, wash and separate the layers, then wash the lower layer with 5% ammonia solution, remove the lower organic phase, dry with anhydrous sodium sulfate, filter to remove the desiccant, concentrate the organic phase to obtain a white solid, After vacuum drying, 17.6 g of 1,1,5,6-isopropylidene-D-mannitol was obtained.
Embodiment 2
[0043] Example 2: Preparation of 1,1,5,6-isopropylidene-D-mannitol
[0044] Add 80 g of zinc chloride and 400 g of acetone into the reaction flask, stir at 30° C. for 30 min, then add 20 g of D-mannitol, and continue stirring for 2 h. After the reaction was completed, 300 mL of saturated sodium chloride solution was added, extracted with 200 mL*2 of dichloromethane, allowed to stand and separated, and the organic phases were combined. Add 300mL of 5% ammonia solution to the organic phase, wash and separate the layers, then wash the lower layer with 5% ammonia solution, remove the lower organic phase, dry with anhydrous sodium sulfate, filter to remove the desiccant, concentrate the organic phase to obtain a white solid, After vacuum drying, 18.1 g of 1,1,5,6-isopropylidene-D-mannitol was obtained.
Embodiment 3
[0045] Embodiment 3: preparation isopropylidene glycerol
[0046] Add 7 g of sodium bicarbonate, 17 g of 1,2,5,6-isopropylidene-D mannitol (intermediate 2), and 250 mL of water into the reaction flask, and stir to dissolve. Add 22 g of sodium periodate to the reaction flask under ice bath, complete the addition within 1 h, and react at room temperature for 2 h. Add 10 g of sodium chloride, stir to dissolve, remove the insoluble matter by filtration, add 10.5 g of sodium borohydride to the filtrate in an ice bath, and complete the addition within 1 h (pay attention to the temperature change of the reaction solution during the addition process to prevent the reaction solution from boiling), room temperature Reaction 2h. The reaction solution was extracted with 100 mL*3 of dichloromethane, and the organic phases were combined and dried over anhydrous sodium sulfate. The desiccant was removed by filtration, and the organic phase was concentrated under reduced pressure to obtain a c
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap