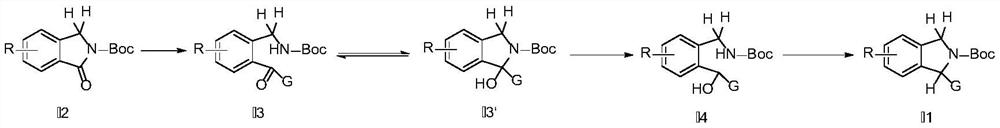
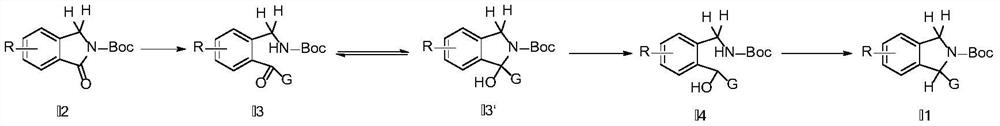
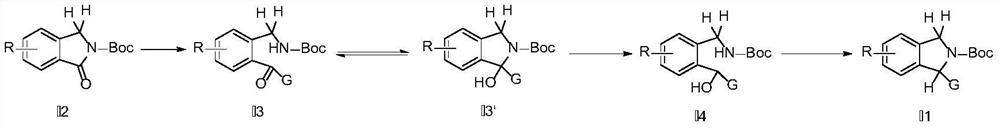
Synthesis method of substituted isoindoline
A synthesis method and isoindoline technology are applied in the field of substituted isoindoline synthesis, can solve the problems of weak reactivity, large steric hindrance, inability to achieve, etc., and achieve the effects of good stability, simple operation, and reduced routes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Preparation method of 1-isopropylisoindoline-2-carboxylic acid tert-butyl ester The structure of 1-isopropylisoindoline-2-carboxylic acid tert-butyl ester is shown in formula 1-1, and the specific steps are as follows:
[0029]
[0030] 1.1 Preparation of oxoisoindoline-2-formic acid tert-butyl ester shown in formula 2-1
[0031]
[0032] Dissolve isoindolin-1-one (758mg, 5.69mmol) in 10ml THF, add (Boc) 2 O (2.5g, 11.39mmol), Et 3 N (1.73g, 17.08mmol), DMAP (69.55mg, 0.57mmol), heated up to 45°C and reacted for 2 hours, TLC followed the reaction, quenched with 20ml of water after the reaction was completed, and extracted with ethyl acetate (2×20ml) , the organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, concentrated, and purified by column chromatography to obtain 1.37g light yellow solid, namely the tert-butyl 1-oxoisoindoline-2-carboxylate (yield: 99%). LC-MS(ESI+):m / z 489(2M+Na) + ; 1 H NMR (300MHz, CDCl 3 ) δ 7
Embodiment 2
[0042] The preparation method of embodiment 2 (p-tolyl) isoindoline-2-formic acid tert-butyl ester
[0043] (P-tolyl) isoindoline-2-formic acid tert-butyl ester is shown in formula 1-2, and concrete steps are as follows:
[0044]
[0045] The preparation of (2-(4-methylbenzoyl) benzyl) tert-butyl carbamate shown in 2.1 formula 3-2
[0046]
[0047] Under nitrogen protection, 1-bromo-4-methylbenzene (73mg, 0.43mmol) was dissolved in 5ml of tetrahydrofuran, the reaction system was cooled to -78°C, and a solution of n-butyllithium (0.2mL, 2.5M) in tetrahydrofuran was slowly added , after the dropwise addition, react at -78°C for 1 h, then dissolve 1-oxoisoindoline-2-carboxylic acid tert-butyl ester (100 mg, 0.43 mmol) shown in formula 2-1 in 1 ml of tetrahydrofuran, and Slowly added dropwise to the above reaction system, quenched with 10 ml of saturated aqueous ammonium chloride solution after the reaction was completed, and extracted with ethyl acetate (3×10 ml), the organic
Embodiment 3
[0054] Example 3 The preparation method of (pyridin-3-yl) isoindoline-2-carboxylic acid tert-butyl ester (pyridin-3-yl) isoindoline-2-carboxylic acid tert-butyl ester is shown in formula 1-3, Specific steps are as follows:
[0055]
[0056] 3.1 Preparation of (2-nicotinoylbenzyl) tert-butyl carbamate shown in formula 3-3
[0057]
[0058] Under nitrogen protection, 3-bromopyridine (68mg, 0.43mmol) 1-oxoisoindoline-2-carboxylic acid tert-butyl ester (100mg, 0.43mmol) was dissolved in 5ml THF, and the reaction system was cooled to -78°C. Add n-BuLi (0.2mL, 2.5M) slowly, react at -78°C for 1h after the dropwise addition, and then add tert-butyl 1-oxoisoindoline-2-carboxylate (100mg , 0.43mmol) was dissolved in tetrahydrofuran (1 ml) and slowly added to the above reaction system, after the reaction was completed, 10 ml of aqueous solution was added to quench, and extracted with ethyl acetate (3 × 10 ml), the organic phase was dried with anhydrous sodium sulfate, Concentrate a
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap