Synthesis method of hydroxyl-substituted styrene compound and synthesis method of photoresist resin monomer
A synthetic method and styrene-based technology, which is applied in the synthesis of styrene-based compounds and the synthesis of photoresist resin monomers, can solve the problems of low yield and low purity, and achieve the effect of high yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
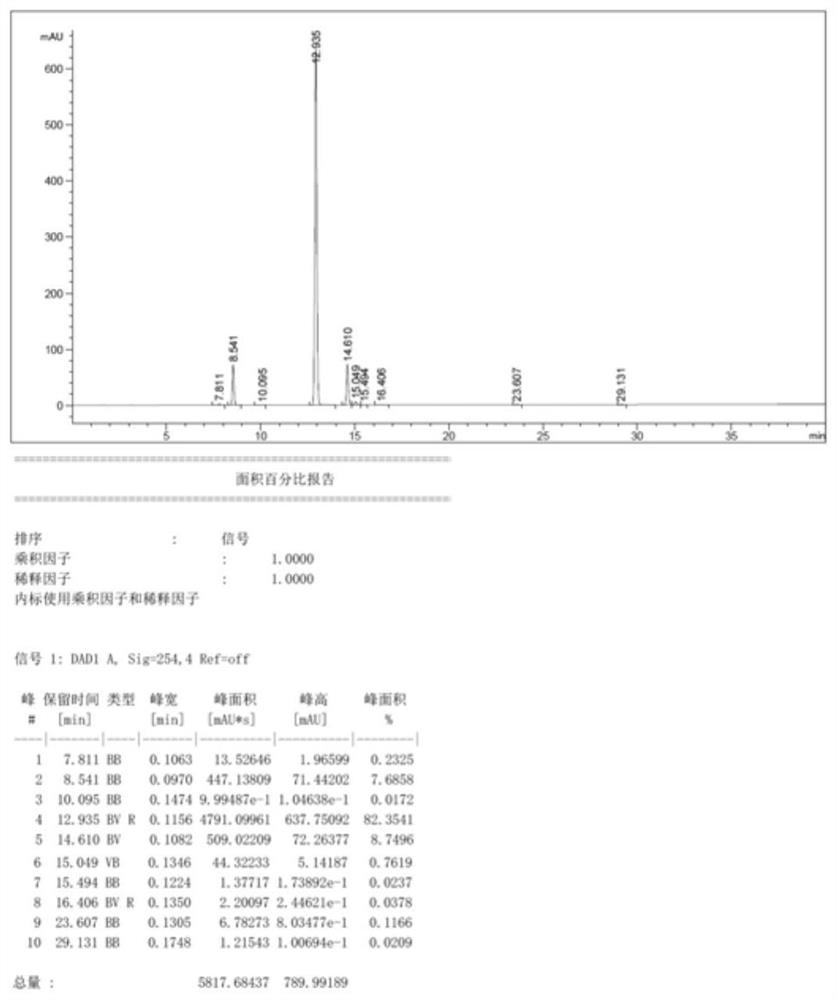
[0069] Add 4450g tetrahydrofuran to a 10L four-necked bottle, N 2 Replacement, start mechanical stirring, add 4400g methyltriphenylphosphine bromide, cool down to -5~-10℃, add 1378g potassium tert-butoxide in batches, stir for 2h after adding, then cool down to -65~-75℃ , add 500g of 2-hydroxybenzaldehyde dropwise, after the dropwise addition, stir for 2 hours and then rise to room temperature (20-25°C) and stir overnight, sample analysis, HPLC purity schematic diagram see figure 1 (raw material<0.1%), HPLC purity 82%.
[0070] Cool the reaction solution to -5~-10°C, then add 1000g of water to quench, add 1800g of 10% HCl after quenching to adjust the pH=7~8, separate the liquid, add 2g of phenothiazine to the organic phase and reduce it at 30~35°C After concentrating, add 2000g of dichloromethane to dissolve, then add anhydrous magnesium sulfate and stir until clarified, filter, filter cake with 200g of dichloromethane, and put the filtrate into the next step. The HPLC purity i
Embodiment 2
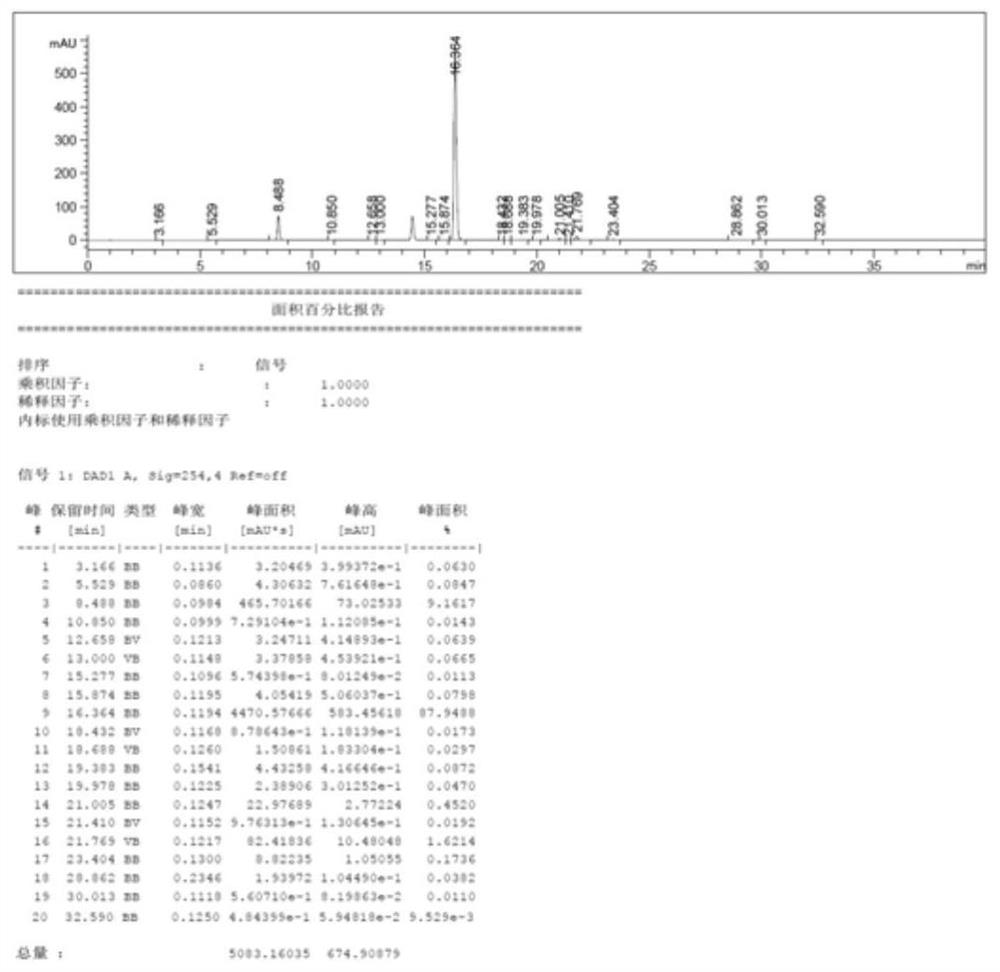
[0074] Add tetrahydrofuran to a 10L four-neck flask, N 2 Replacement, turn on the mechanical stirring, add methyltriphenylphosphine bromide, cool down to -5°C, add potassium tert-butoxide in batches, stir for 2 hours after the addition, then cool down to -70°C, add dropwise 500g of 2-hydroxybenzene Formaldehyde, stirred for 2 hours after the dropwise addition, raised to room temperature and stirred overnight, sampled and analyzed, the HPLC purity was 81%, wherein, the molar ratio of 2-hydroxybenzaldehyde to methyltriphenylphosphine bromide was 1:1, and tetrahydrofuran The mass ratio is 1:15, and the molar ratio to potassium tert-butoxide is 1:1.
[0075] Cool the reaction solution to -7°C, then add 1000g of water to quench, add 10% HCl after quenching to adjust pH=7.5, separate the liquid, add 2g of phenothiazine to the organic phase and concentrate under reduced pressure (200Pa) at 35°C, the concentration is complete Add 2000g of dichloromethane to dissolve, then add anhydrous
Embodiment 3
[0079] Add tetrahydrofuran to a 10L four-neck flask, N 2 Replacement, turn on the mechanical stirring, add methyl triphenylphosphine bromide, cool down to -8°C, add potassium tert-butoxide in batches, stir for 2 hours after the addition, then cool down to -65°C, add dropwise 500g of 2-hydroxybenzene Formaldehyde, stirred for 2 hours after the dropwise addition, rose to room temperature and stirred overnight, sampled and analyzed, the HPLC purity was 80%, wherein, the molar ratio of 2-hydroxybenzaldehyde to methyltriphenylphosphine bromide was 1:5, and tetrahydrofuran The mass ratio is 1:5, and the molar ratio to potassium tert-butoxide is 1:6.
[0080] Cool the reaction solution to -5°C, then add 1000g of water to quench, add 10% HCl after quenching to adjust the pH=7, separate the liquids, add 2g of phenothiazine to the organic phase and concentrate under reduced pressure (50Pa) at 30°C, and the concentration is complete Add 2000g of dichloromethane to dissolve, then add anhydr
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap