Amphiphilic alpha-helical polypeptide for inhibiting PD-L1 and application of amphiphilic alpha-helical polypeptide
A PD-L1 and amphiphilic technology, which is applied in the field of preparing anti-tumor drugs, can solve the problems of no related research, and achieve the effect of reducing immunogenicity, eliminating immunosuppression, and preventing immune escape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
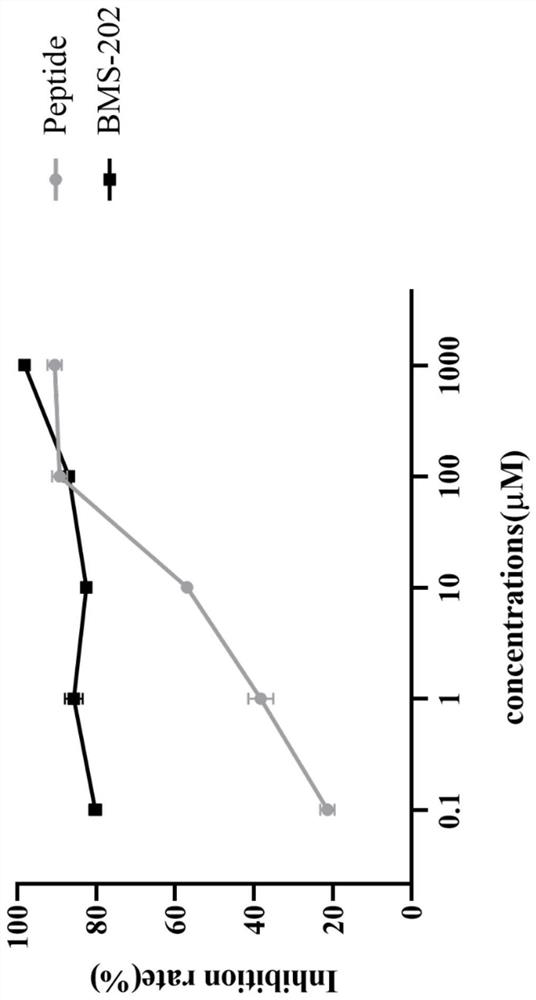
[0031] In vitro blocking of PD-1 / PD-L1 interaction experiments by the polypeptides described in Example 1
[0032] 1. Medication
[0033] Before the experiment, the peptides to be tested and the positive control drug (BMS-202) were dissolved in Diluent Buffer and DMSO, respectively, to prepare a stock solution with a concentration of 100 mM, and then diluted with Diluent Buffer to 10 mM, 1000 μM, 100 μM, 10 μM, and 1 μM, respectively. The test solution was frozen at -20°C. The Tag1-PD-L1 (0.1mg / mL, 1.845μM, 35μL) and Tag2-PD-1 (0.5mg / Ml, 27.8μM, 25μL) protein working solutions in the HTRF kit were diluted with Diluent Buffer to the final concentration. 25nM and 250nM, aliquoted into sample tubes. The energy donor Anti-Tag1-Eu in the HTRF kit 3+ (25μL), energy donor Anti-Tag2-XL665 (100μL) stock solution was diluted 100-fold and 25-fold with Detection Buffer respectively, aliquoted into sample tubes, and stored at -80°C. Freeze and thaw repeatedly.
[0034] 2. Experimen
Embodiment 2
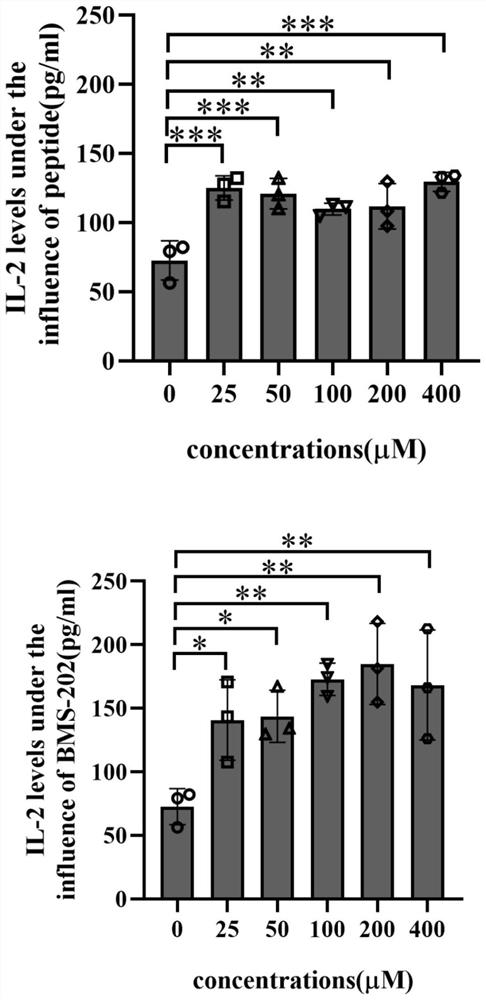
[0044] Example 2 Jurkat cells were co-cultured with MDA-MB-231 cells, and IL-2 secretion in cell supernatant was detected by Elisa method
[0045] 1. Medium configuration: Dissolve 20μg human IFN-γ in 1mL ddH 2 O, prepared into a 20 μg / mL stock solution, dispensed into multiple EP tubes, and frozen in -80 °C ultra-low temperature refrigerator. Human-derived IFN-γ stock solution was prepared into DMEM complete medium containing 10 ng / mL human-derived IFN-γ using DMEM complete medium, and aliquoted for cryopreservation. Similarly, Phorbol 12-myristate 13-acetate (PMA) and Phytohemagglutinin (PHA) were formulated to contain 50 ng of RPMI-1640 complete medium. / L PMA and 1 μg / mL PHA in RPMI-1640 complete medium, aliquoted for cryopreservation.
[0046] 2. Cell culture: Breast cancer MDA-MB-231 cells were cultured in DMEM complete medium containing 10 ng / mL human IFN-γ for 24 hours to induce high expression of PD-L1 in MDA-MB-231 cells, and Jurkat cells in Jurkat cell dif
Embodiment 3
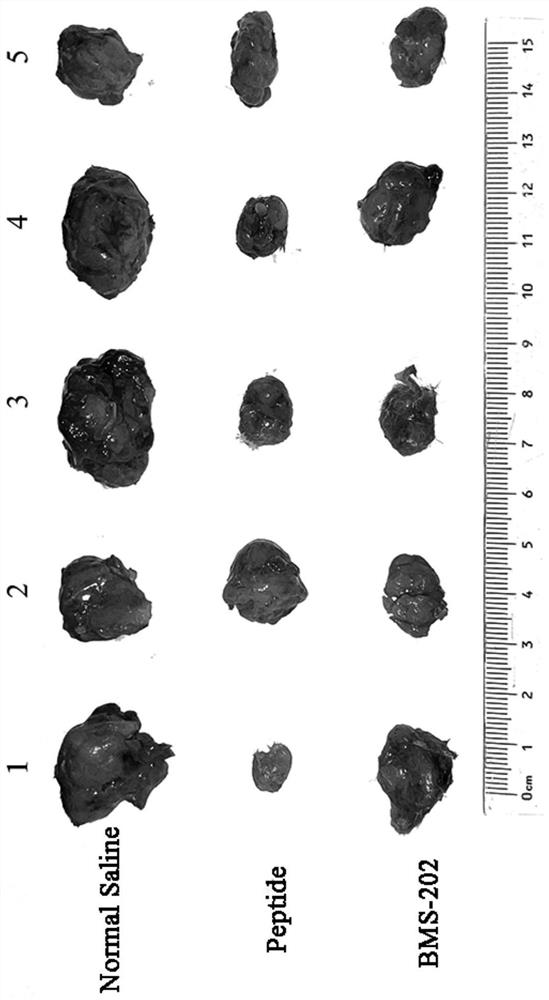
[0064] Example 3 Using the polypeptide as an example to conduct an in vivo antitumor activity experiment
[0065] The anti-tumor activity of the polypeptide in vivo was investigated by establishing a 4T1-tumor-bearing mouse model. The methods and results are as follows:
[0066] 1. Materials and grouping: 15 female balb / c mice aged 6-8 weeks were ordered from the Experimental Animal Center of Jiangsu University. Mice were randomly divided into 3 groups with 5 mice in each group: polypeptide group (20 mg / kg), positive control BMS-202 (10 mg / kg), model group (physiological saline). Dissolve 20 mg of polypeptide in 10 mL of physiological saline to prepare a 2 mg / mL polypeptide solution, which is then frozen in aliquots. Take 1mL of 25mg / mL clear DMSO stock solution and add it to 9mL of 20% SBE-β-CD saline solution, mix well, dissolve 10mg BMS-202 in this solution to obtain 1mg / mL BMS-202 solution, divide Store frozen. Dissolve 50 μg of mouse-derived IFN-γ in 0.5 mL of
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap