Boron-doped carbon quantum dot containing transition metal as well as preparation method and application of boron-doped carbon quantum dot
A transition metal, carbon quantum dot technology, applied in chemical instruments and methods, nano-carbon, material excitation analysis, etc., can solve problems such as unreliable detection results and signal interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
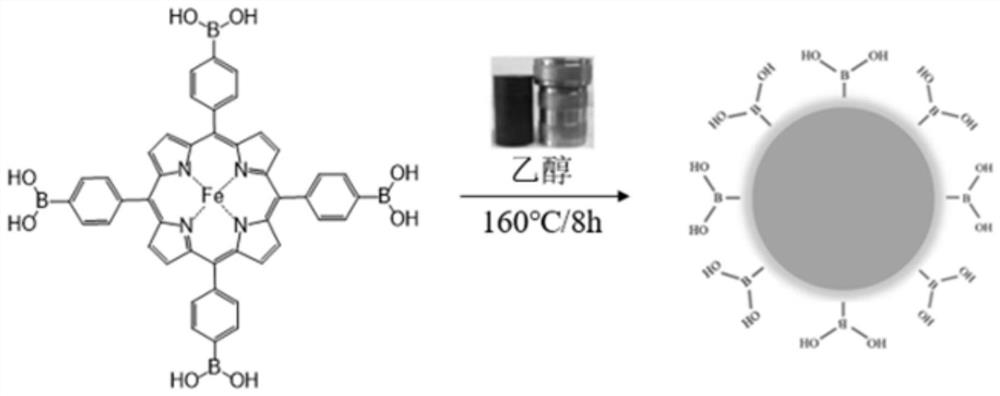
[0028] (1) Add 25 mg of porphyrin tetraphenylboronic acid and 20 mg of ferrous chloride into a 200 mL beaker, then add 100 mL of absolute ethanol, 0.5 mL of 68% nitric acid aqueous solution, and 0.25 mL of ethylenediamine, and then ultrasonicate for 10 min to make The tetraphenylboronic acid porphyrin was completely dissolved.
[0029] (2) The mixed solution obtained in step (1) was transferred to a 200mL reaction kettle, and the reaction kettle was put into a temperature-controlled oven, reacted at a set temperature, and naturally cooled to room temperature after the reaction was completed. The set reaction temperature was 160°C, and the set reaction time was 8h.
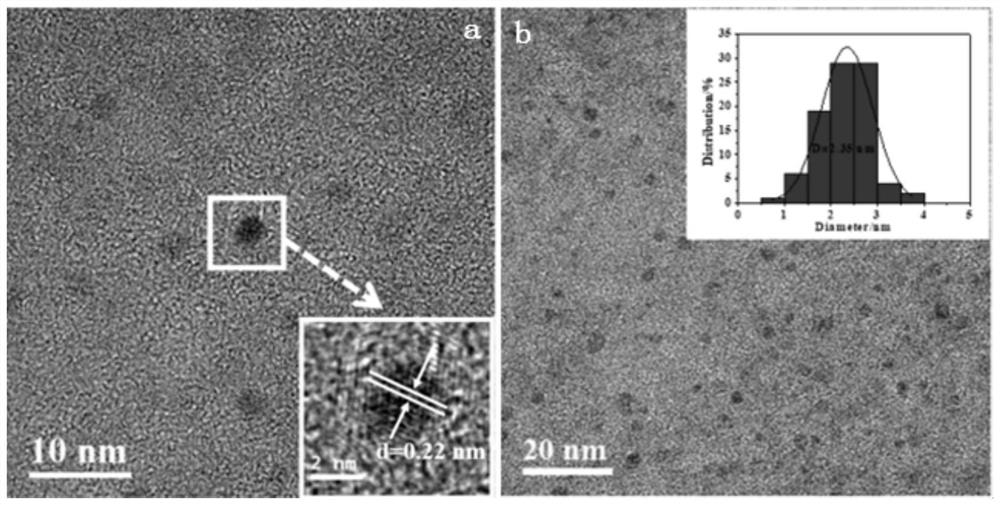
[0030] (3) Centrifuge the reaction solution, discard the solids precipitated at the bottom, dialyze the supernatant in an aqueous solution with a dialysis bag with a molecular weight cut-off of 1000 Da for 72 hours, and then place it at -60 °C for lyophilization for 48 hours to finally obtain the target carbon quantum
Embodiment 2
[0032] (1) Add 46 mg of B-1,10-phenanthroline-5-ylboronic acid and 10 mg of ferrous chloride into a 50 mL beaker, then add 25 mL of DMF, 0.1 mL of 68% nitric acid aqueous solution, 0.05 mL of ethyl acetate Diamine, followed by sonication for 20 min to completely dissolve the starting material.
[0033] (2) The mixed solution obtained in step (1) was transferred to a 50mL reaction kettle, the reaction kettle was put into a temperature-programmed oven, reacted at a set temperature, and naturally cooled to room temperature after the reaction was completed. The set reaction temperature was 160°C, and the set reaction time was 8h.
[0034] (3) Centrifuge the reaction solution, discard the solid precipitated at the bottom, dialyze the supernatant in an aqueous solution with a dialysis bag with a molecular weight cut-off of 500 Da for 72 hours, and then place it at -60 °C for lyophilization for 48 hours to finally obtain the target carbon quantum dots .
Embodiment 3
[0036] (1) Add 41 mg of quinoline-5-boronic acid and 10 mg of ferrous chloride into a 50 mL beaker, then add 25 mL of DMF, 0.1 mL of a 68% nitric acid aqueous solution, and 0.05 mL of ethylenediamine, followed by ultrasonication for 20 min to make the raw materials completely dissolved.
[0037] (2) The mixed solution obtained in step (1) was transferred to a 50mL reaction kettle, the reaction kettle was put into a temperature-programmed oven, reacted at a set temperature, and naturally cooled to room temperature after the reaction was completed. The set reaction temperature was 160°C, and the set reaction time was 8h.
[0038] (3) Centrifuge the reaction solution, discard the solid precipitated at the bottom, dialyze the supernatant in an aqueous solution with a dialysis bag with a molecular weight cut-off of 500 Da for 72 hours, and then place it at -60 °C for lyophilization for 48 hours to finally obtain the target carbon quantum dots .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap