Method and apparatus for verifying a determined cardiac event in a medical device based on detected variation in hemodynamic status
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
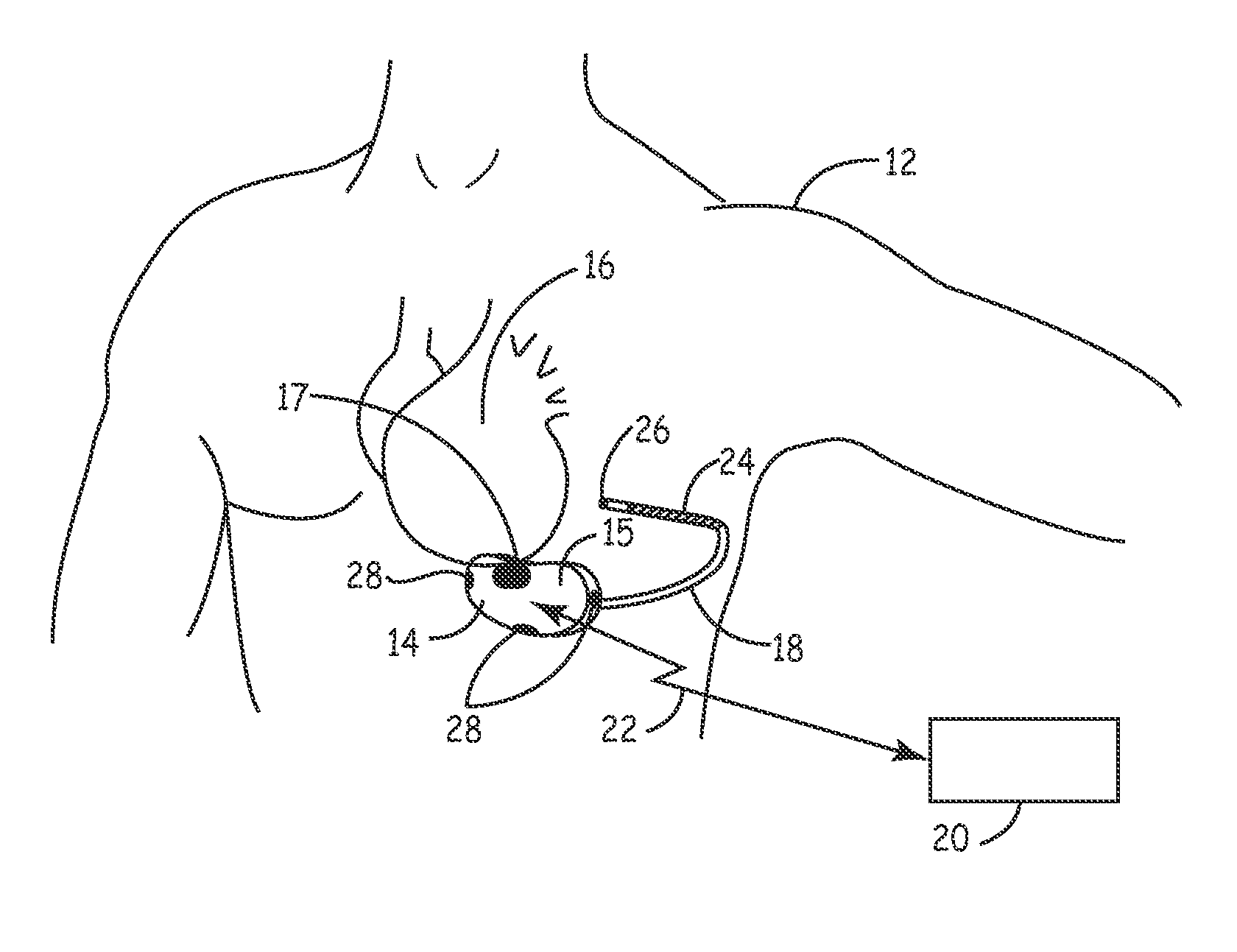
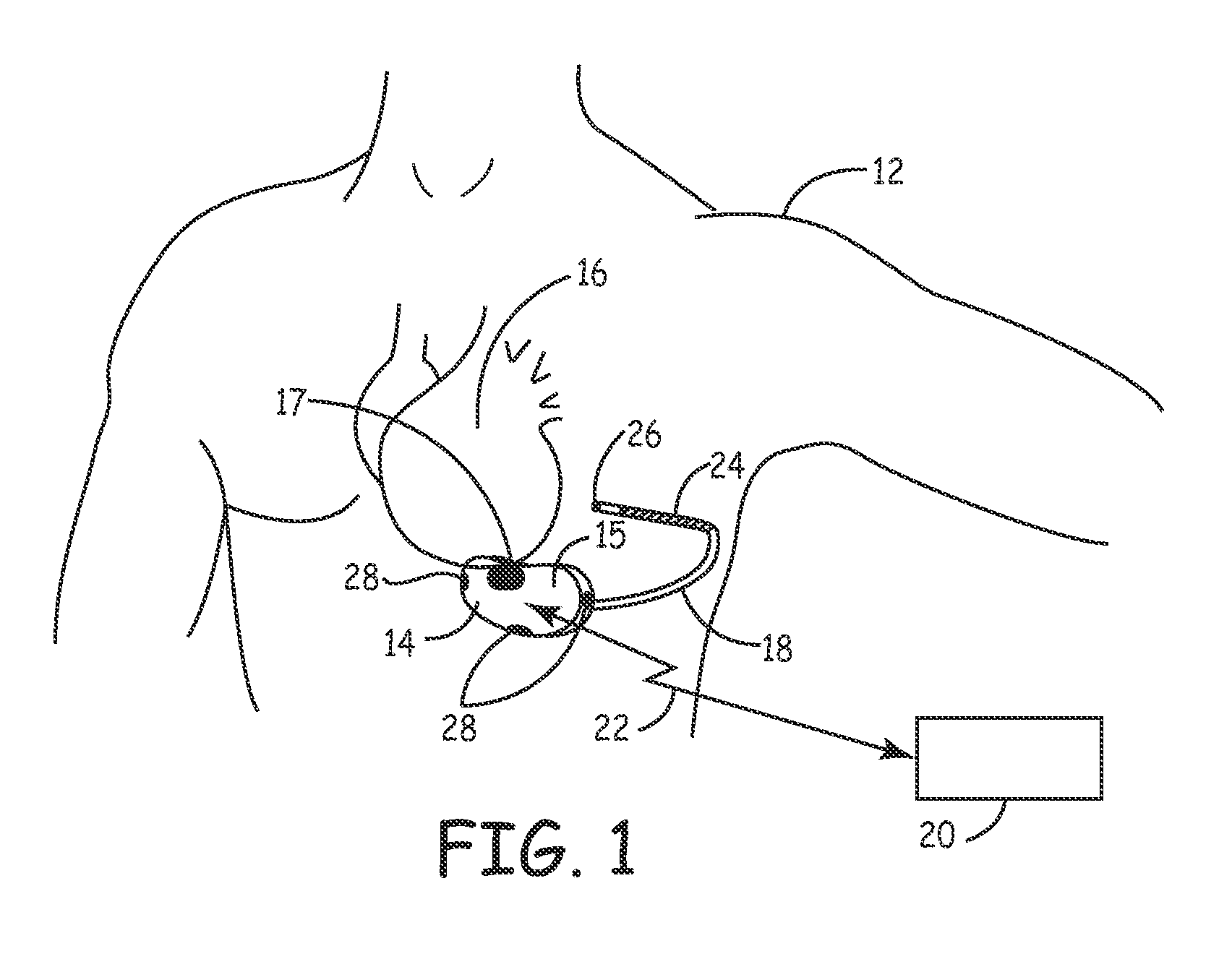
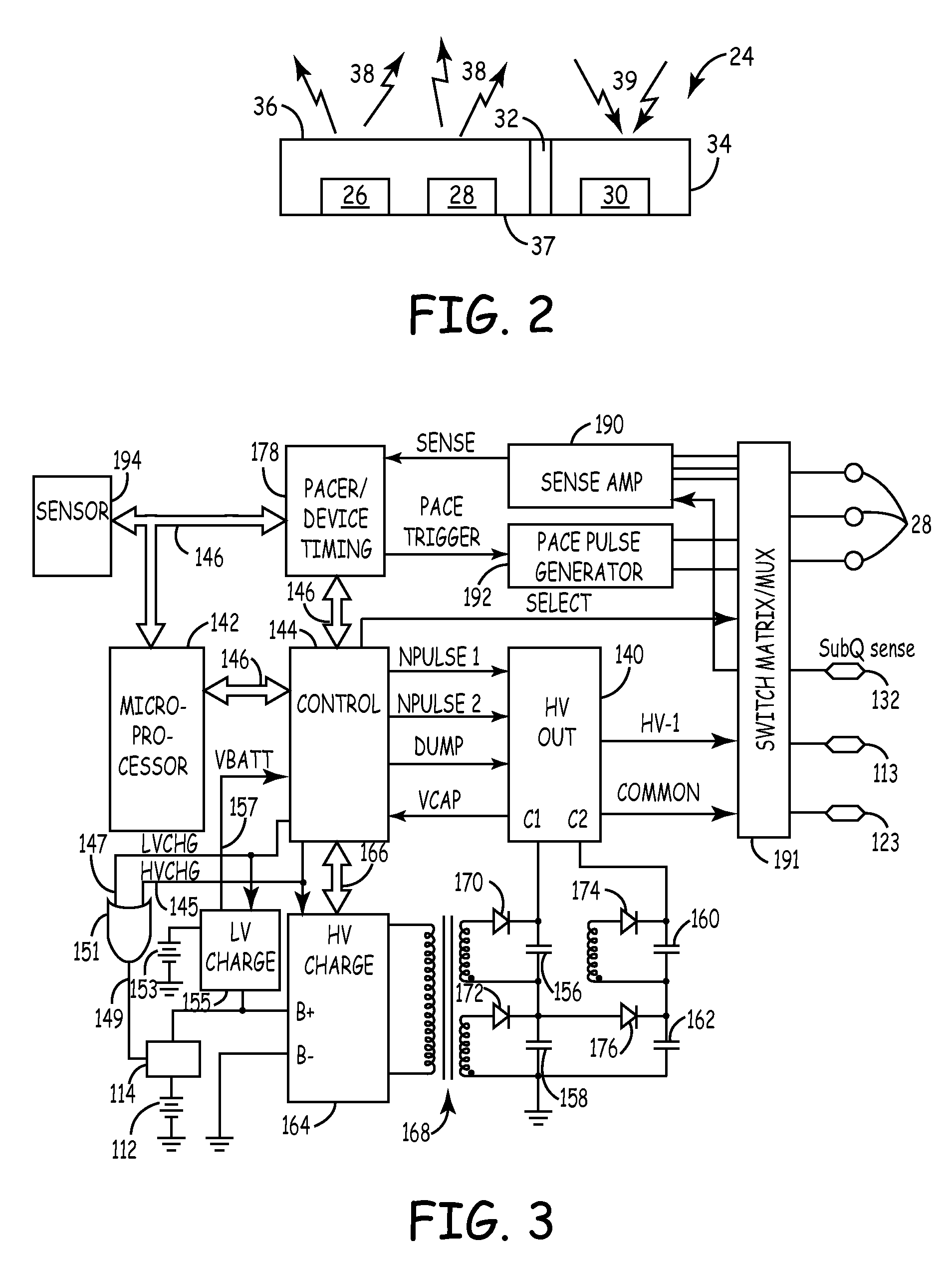
[0023]FIG. 1 is a schematic diagram of an exemplary medical device in which the present invention may be usefully practiced. As illustrated in FIG. 1, the present invention may be utilized in an implantable medical device 14 that includes a housing 15 containing circuitry for operating device 14 that is subcutaneously implanted in a patient, outside the ribcage of patient 12, anterior to the cardiac notch, for example. According to an embodiment of the present invention, housing 15 may be implanted in the pectoral region of the patient 12. Further, device 14 may include a subcutaneous sensing and cardioversion / defibrillation therapy delivery lead 18 coupled to the device 14 that is tunneled subcutaneously into a location adjacent to a portion of a latissimus dorsi muscle of patient 12. Specifically, lead 18 is tunneled subcutaneously from the median implant pocket of device 14 laterally and posterially to the patient's back to a location opposite the heart such that the heart 16 is d
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap