Opsin-Binding Ligands, Compositions and Methods of Use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
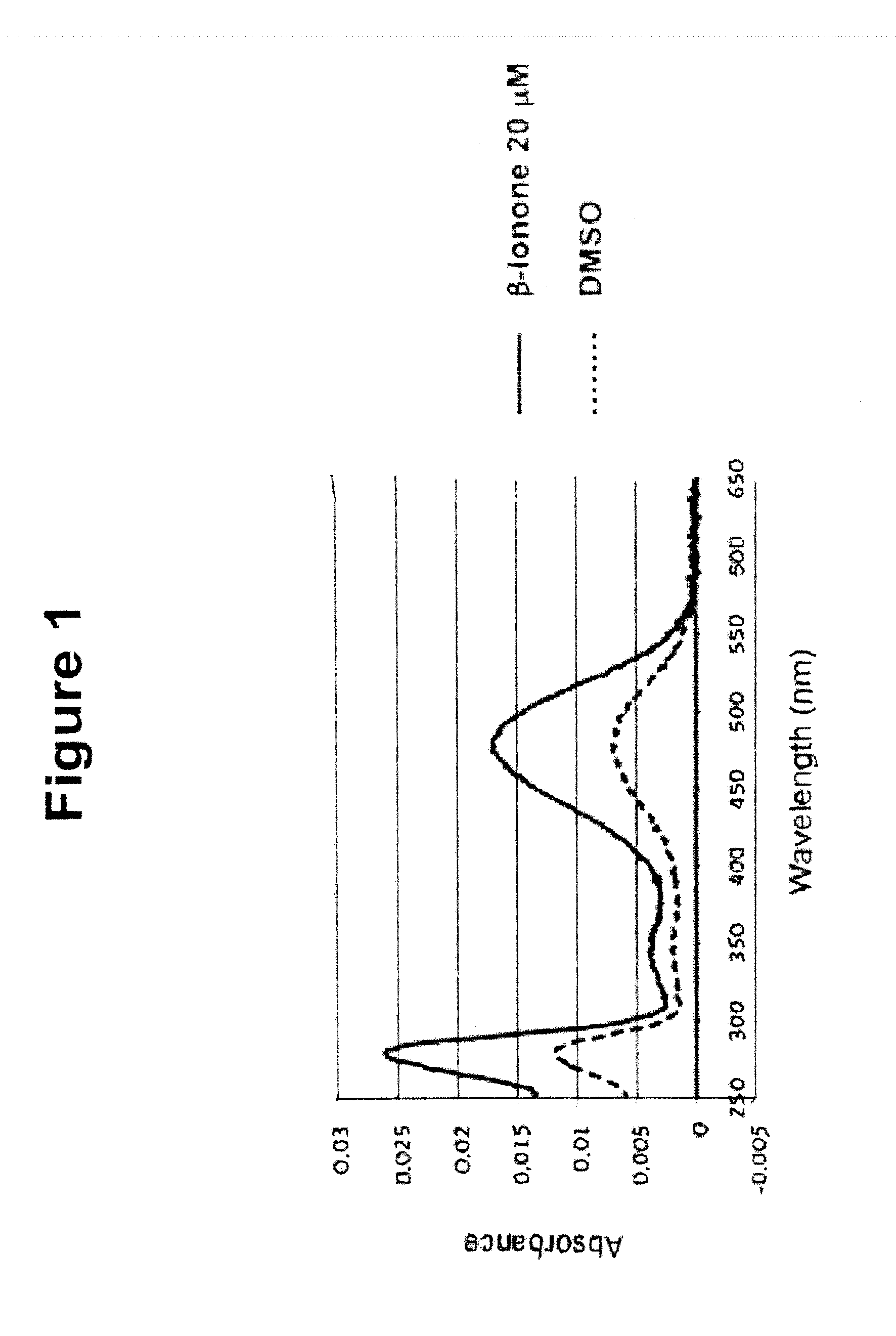
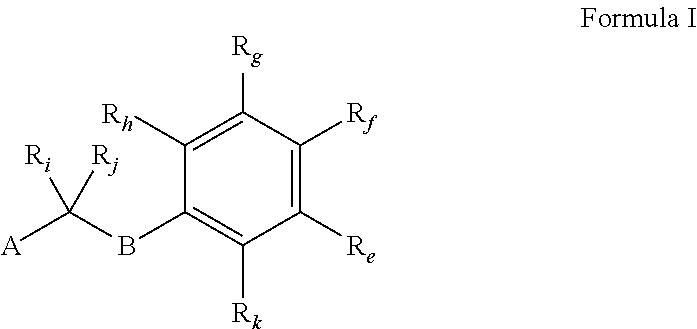
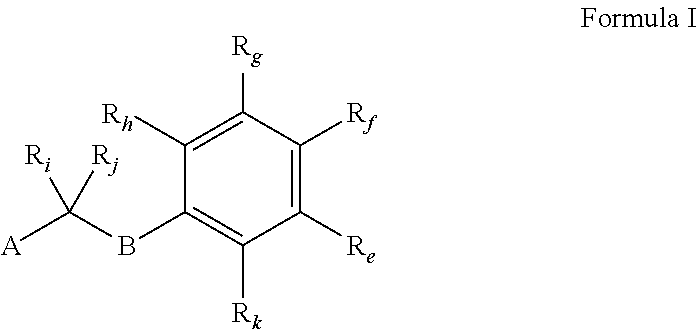
Image
Examples
Example
Example 1
3-(trifluoromethyl)-N-((2,6,6-trimethylcyclohex-1-enyl)methyl)aniline hydrochloride
Example
Example 1a
3-(trifluoromethyl)-N-((2,6,6-trimethylcyclohex-1-enyl)methyl)aniline
[0420]To a stirred solution of 2,6,6-trimethylcyclohex-1-enecarbaldehyde (152 mg, 1.0 mmol) in methanol (3 mL) was added 3-(trifluoromethyl)aniline (161 mg, 1.0 mmol). To the stirred mixture was added acetic acid (0.1 mL) followed by sodium cyanborohydride (188 mg, 3.0 mmol). The reaction was stirred at room temperature for 3 hours and then the reaction was quenched by addition of saturated aqueous ammonium chloride (5 mL). The methanol was evaporated under reduced pressure and the residue was diluted with ethyl acetate (40 mL). The organic phase was washed with water (30 mL×2) and brine (30 mL), dried over anhydrous sodium sulfate and concentrated under reduced pressure. The residue was purified by silca gel column chromatography (eluent: petroleum ether / ethyl acetate=100 / 1) to afford the title compound as a colorless oil (200 mg, Yield: 67%). Rf=0.8 (30:1 petroleum ether / ethyl acetate); 1H NMR (400 M
Example
Example 2
3-methyl-N-((2,6,6-trimethylcyclohex-1-enyl)methyl)aniline
[0422]To a stirred solution of 2,6,6-trimethylcyclohex-1-enecarbaldehyde (152 mg, 1.0 mmol) in methanol (3 mL) was added m-toluidine (107 mg, 1.0 mmol), acetic acid (0.1 mL) followed by sodium cyanoborhydride (187 mg, 3.0 mmol). The reaction was stirred at room temperature for 1 hour and then was quenched by addition of saturated aqueous ammonium chloride (5 mL). The methanol was evaporated under reduced pressure and the residue was diluted with ethyl acetate (40 mL). The organic phase was washed with water (30 mL×2) and brine (30 mL), dried over anhydrous sodium sulfate and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (eluent: petroleum ether / ethyl acetate=100 / 1) to afford the title compound as a colorless oil (185 mg, Yield: 76%). Rf=0.7 (20:1 petroleum ether / ethyl acetate); 1H NMR (400 MHz, CDCl3) δ 7.09-7.05 (m, 1H), 6.50 (d, J=7.2 Hz, 1H), 6.42 (s, 1H), 6.4
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electrical conductance | aaaaa | aaaaa |
| Toxicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap