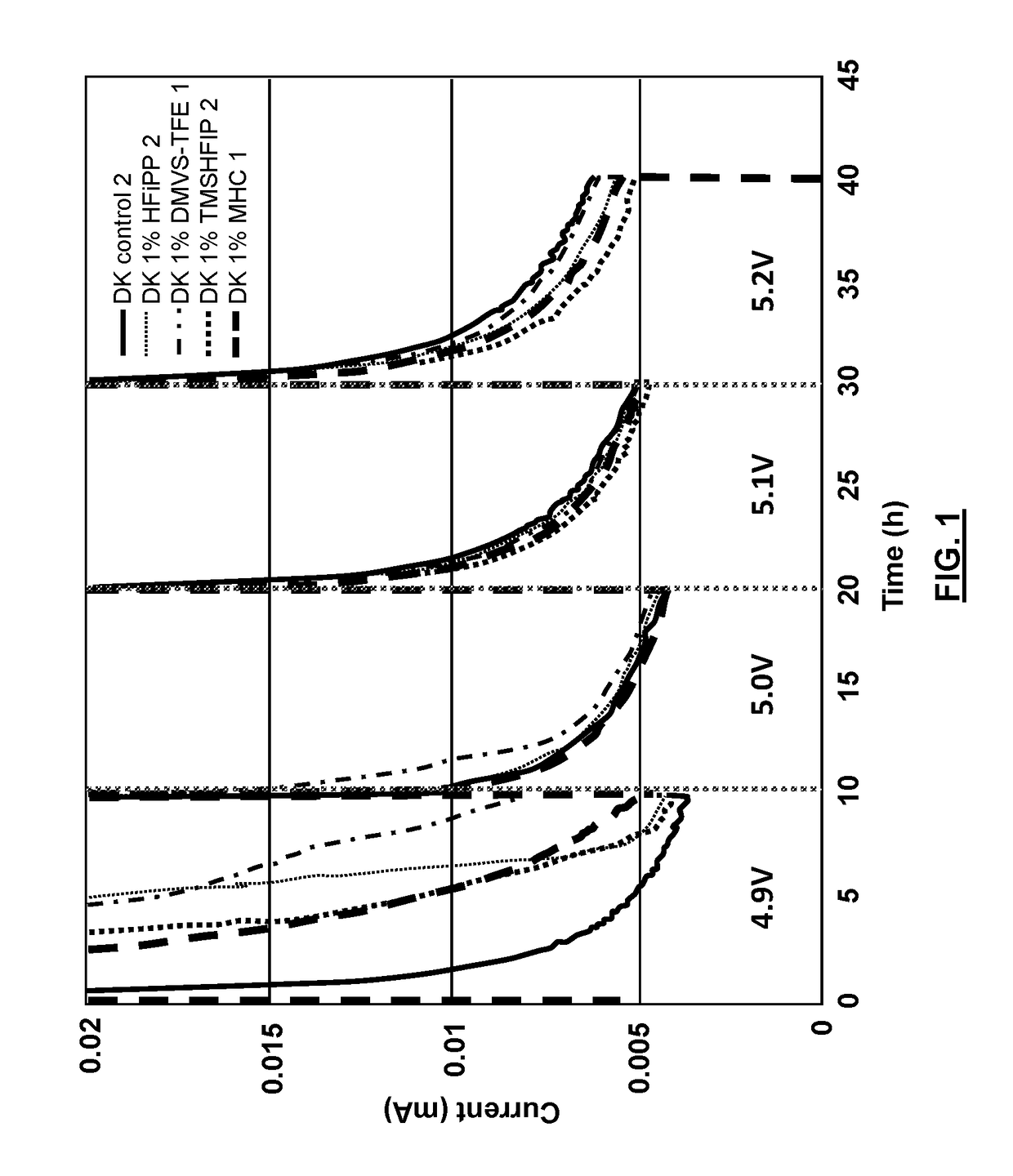
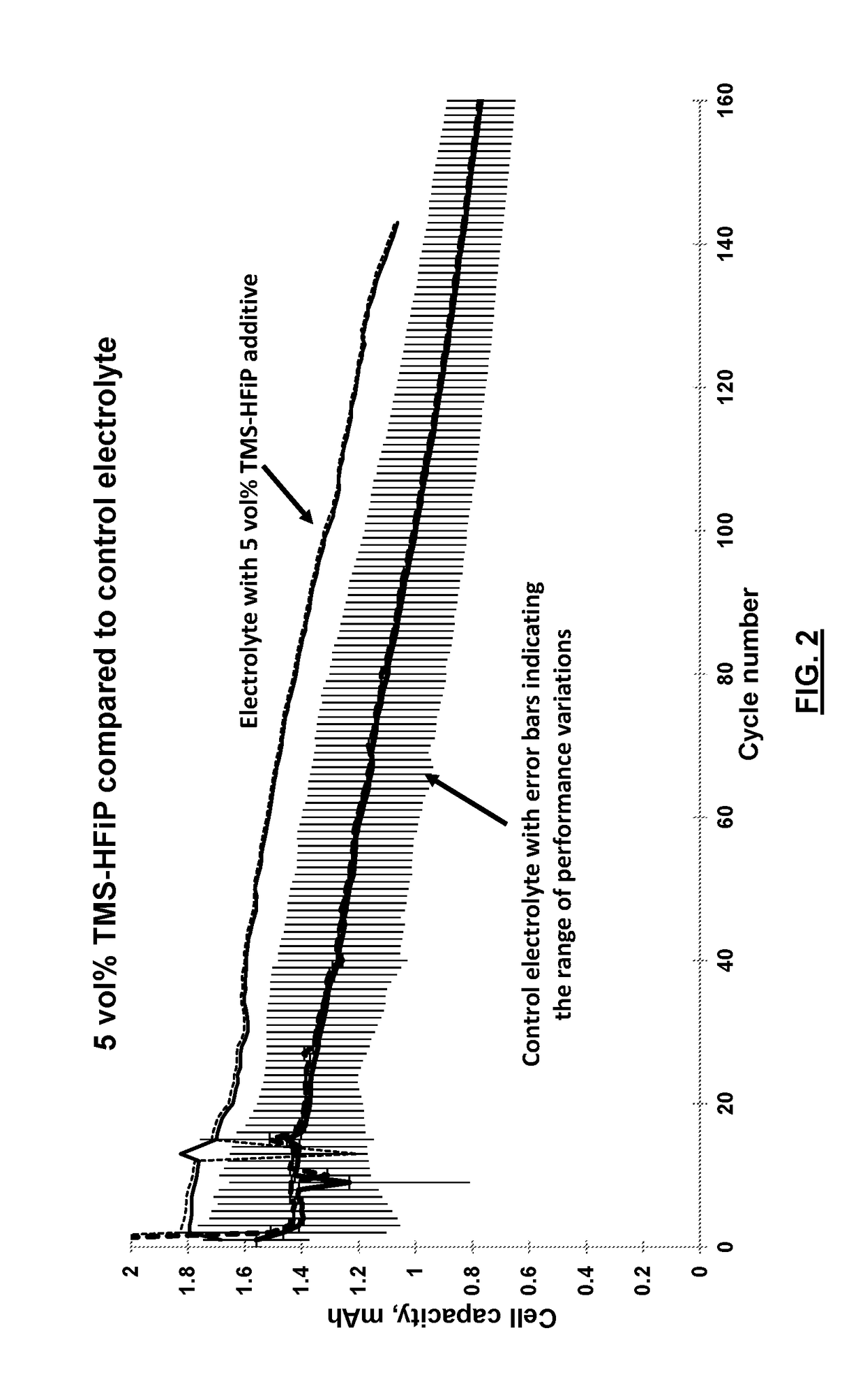
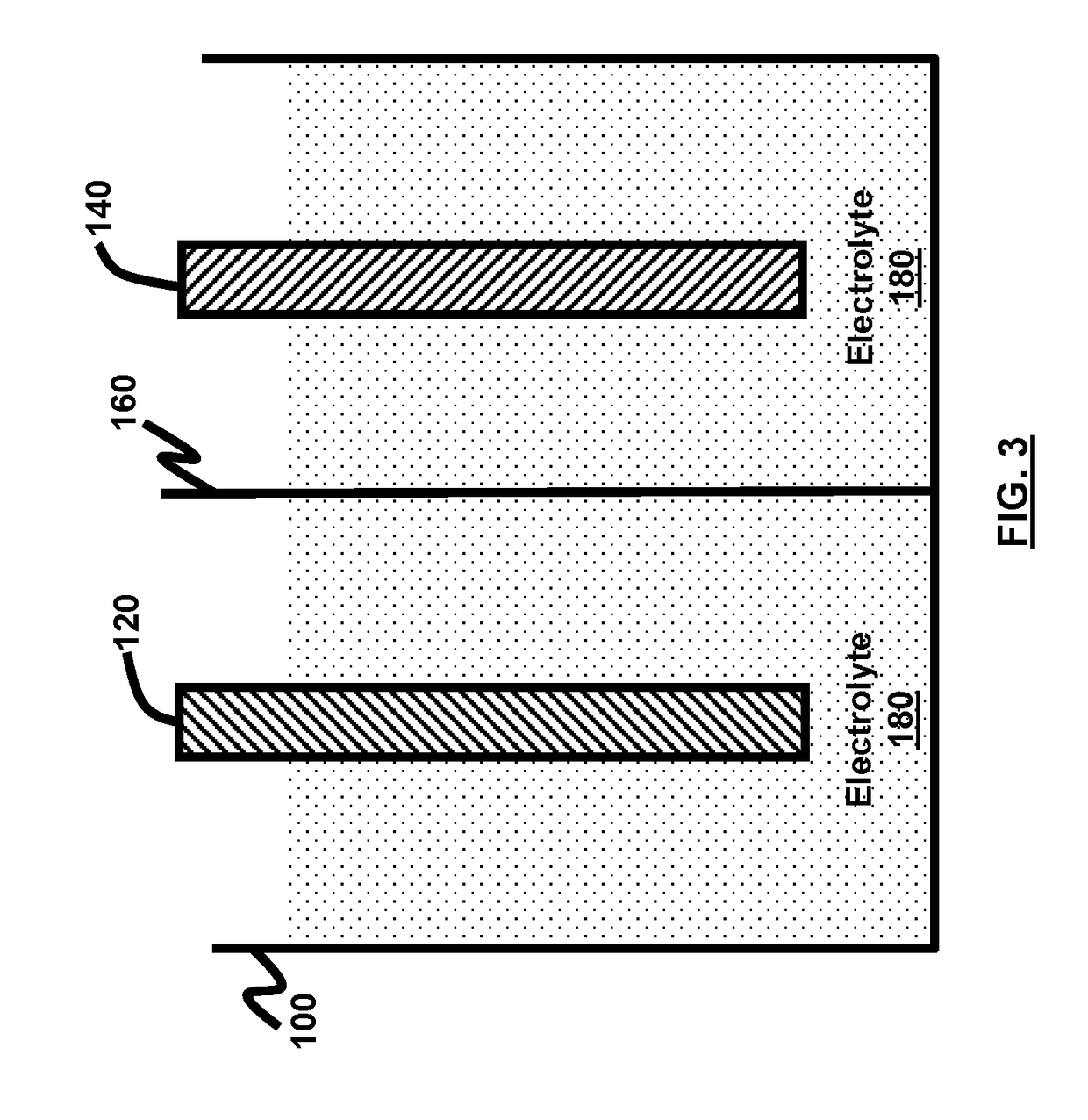
Electrolyte solvents and additives for advanced battery chemistries
a technology of additives and electrolytes, applied in the field of nonaqueous electrolytes, can solve the problems of inability to accurately understand, the cathode surface additive developed for a good cathode surface fails to deliver the expected performance once placed in a full rechargeable cell, and the above-described solvent and/or additives in state-of-the-art electrolytes meet severe challenges
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
of Trimethylsilyl Propargylformate
[0040]
[0041]To a flask containing 32.88 g (0.256 mol) potassium trimethylsilonate (Me3SiOK) is suspended in 100 mL anhydrous diethyl ether, and 25 mL (˜0.26 mol) propargyl chloroformate is added dropwise under stirring. The reaction is exothermic with white precipitation. Upon completion of addition, the solution is heated to reflux and then cooled down. The final product is filtered at room temperature, and filtrate is subject to repeated distillations. Final fractionation yield 80% of final product in the boiling range of 88˜95° C. The structural analysis conducted through gas chromatography-mass spectrometry (GC-MS) confirms the purity of the product to be over 99.9%, and the structure is confirmed by both MS and multi-nuclei nuclear magnetic resonance (NMR) spectroscopy.
example 2
of Trimethylsilyl Hexafluoro-Isopropyl Ether
[0042]
[0043]To a flask containing 0.50 mol LiH suspended in 500 mL diethylether, 0.50 mol of hexafluoro-iso-propyl alcohol is added dropwise under stirring. Upon completion of the addition and releasing of hydrogen, the solution is heated to reflux and then cooled down. 0.51 mol of trimethylsilyl chloride dissolved in 500 mL diethylether is gradually added. The reaction is exothermic, and further heating is applied to reflux the reactants in order to ensure the completion of reaction. The final product is filtered at room temperature, and filtrate is subject to repeated distillations. Final fractionation yields 70% of final product in the boiling range of 80˜85° C. The structural analysis conducted through GC-MS confirms the purity of the product to be over 99.9%, and the structure is confirmed by both MS and multi-nuclei NMR spectroscopy.
example 3
of Dimethylvinylsilyl Hexafluoro-Isopropyl Ether
[0044]
[0045]To a flask containing 5.760 g (0.724 mol) LiH suspended in 500 mL diethylether, 121.0 g (0.724 mol) of hexafluoro-iso-propyl alcohol is added dropwise under stirring. Upon completion of addition and releasing of hydrogen, the solution is heated to reflux and then cooled down. 100 mL (0.724 mol) of dimethylvinylsilyl chloride dissolved in 100 mL diethylether is gradually added. The reaction is exothermic with white precipitation, and further heating is applied to reflux the reactants in order to ensure the completion of reaction. The final product is filtered at room temperature, and filtrate is subject to repeated distillations. Final fractionation yields 58% of final product in the boiling range of 82˜83° C. The structural analysis conducted through GC-MS confirms the purity of the product to be over 99.9%, and the structure is confirmed by both MS and multi-nuclei NMR spectroscopy.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap