Selective porphyrin-catalyzed electrochemical reduction of co2 into co, in particular in water
a porphyrincatalyzed electrochemical and electrochemical reduction technology, which is applied in the direction of organic compounds/hydrides/coordination complex catalysts, physical/chemical process catalysts, iron organic compounds, etc., can solve the problems of poor selective direct electrochemical reduction of co2 /sub>at inert electrodes, low chemical reactivity, etc., and achieve high and tunable selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
of the Electrolyte Solutions
[0138]In a first embodiment, the solvent of the cathodic and anodic electrolyte solutions is water.
[0139]When syngas is produced in this embodiment, the pH of the cathodic electrolyte solution (notably through appropriate choice of buffer) and the potential applied to the cathode may be adjusted so as to tune (or choose) the CO / H2 molar ratio of the produced gas. In this case, the cathodic electrolyte solution preferably comprises a buffer such as a phosphate buffer. The pH of the cathodic electrolyte solution is also typically of between 6.5 and 7.5.
[0140]For producing pure CO, the pH of the cathodic electrolyte solution is preferably of between 6.5 and 7.5, and the cathodic electrolyte solution is advantageously devoid of buffer, in particular it is devoid of phosphate buffer. In this embodiment, the pH of the cathodic electrolyte solution is advantageously adjusted by adding alkali metal salts, preferably hydroxide, carbonate or bicarbonate alkali metal
second embodiment
Solvent of the Electrolyte Solutions
[0141]In a second embodiment, the solvent of the cathodic and anodic electrolyte solutions is an organic medium, such as DMF (dimethylformamide), ACN (acetonitrile), and aqueous mixtures thereof. Preferably, the electrolyte solutions further comprise a proton donor as described above.
[0142]Advantageously, the intensity applied to the cathode is between 2 and 5 A / m2, more preferably between 2.5 and 4 A / m2, even more preferably between 3 and 3.5 A / m2.
[0143]Advantageously, the potential applied to the cathode is between −2.5 V and −0.5 V versus NHE, more advantageously between −2.0 V and −0.5 V versus NHE, more advantageously between −1.5 V and −0.8 V versus NHE, more advantageously between −1.3 V and −0.9 V versus NHE, and the intensity applied to the cathode is advantageously between 2 and 5 A / m2, more preferably between 2.5 and 4 A / m2, even more preferably between 3 and 3.5 A / m2.
DESCRIPTION OF THE DRAWINGS
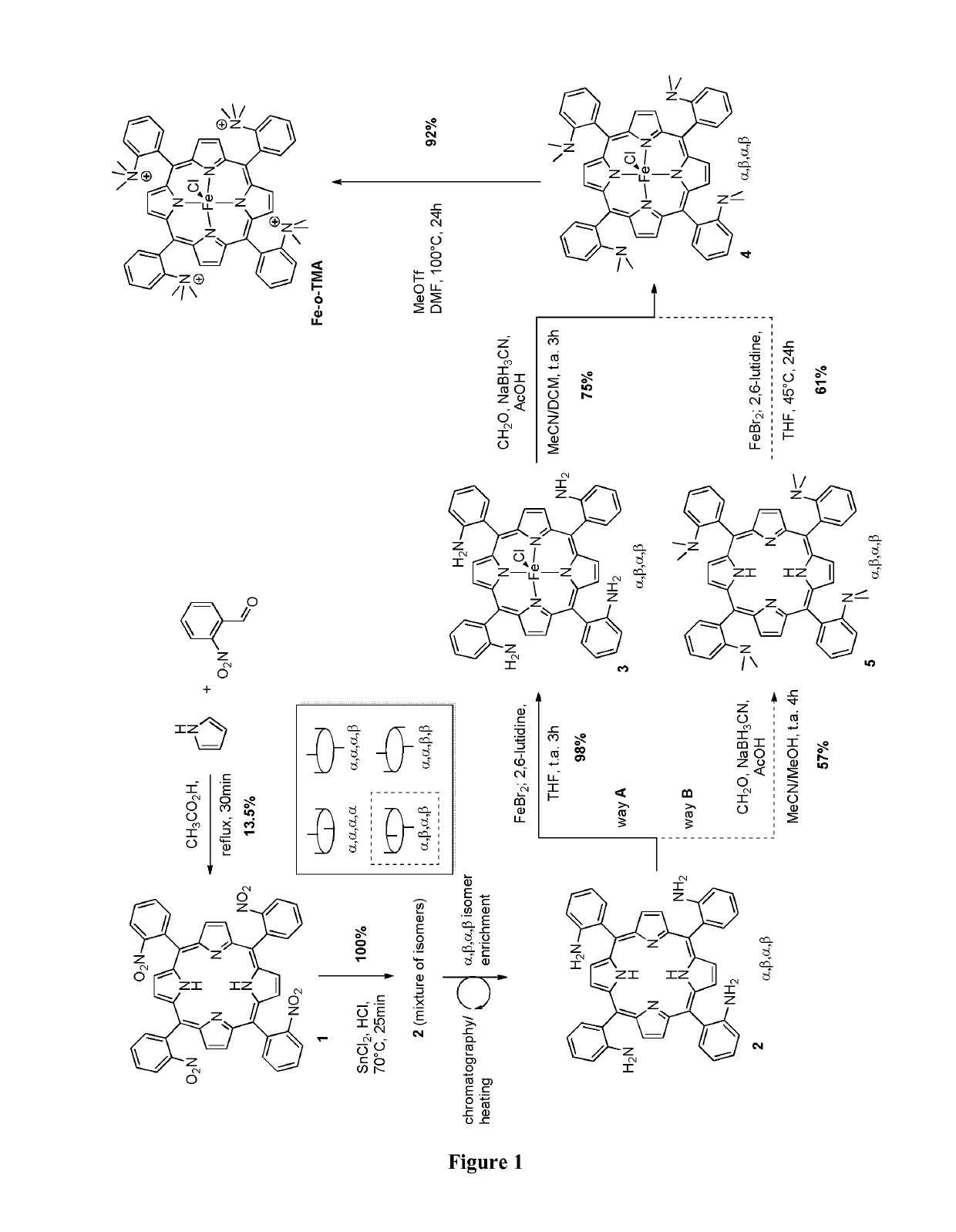
[0144]FIG. 1 represents the synth
example 1
and Characterization of Fe-o-TMA
[0162]Chemicals.
[0163]Dimethylformamide (Acros, >99.8%, extra dry over molecular sieves), the supporting electrolyte NBu4PF6 (Fluka, purriss.) were used as received. All starting materials were obtained from Sigma-Aldrich, Fluka, Acros and Alfa-Aesar, and were used without further purification. CHCl3 and CH2Cl2 were distilled from calcium hydride and stored under an argon atmosphere.
[0164]Materials.
[0165]1H NMR spectra were recorded on a Bruker Avance III 400 MHz spectrometer and were referenced to the resonances of the solvent used. The mass spectra were recorded on a MALDI 4800 TOF / TOF. The elemental analysis were performed by the Microanalysis Service of the Institut de Chimie des Substances Naturelles (ICSN-CNRS), Avenue de la Terrasse, 91198 Gif-sur-Yvette cedex, France.
[0166]Porphyrins.
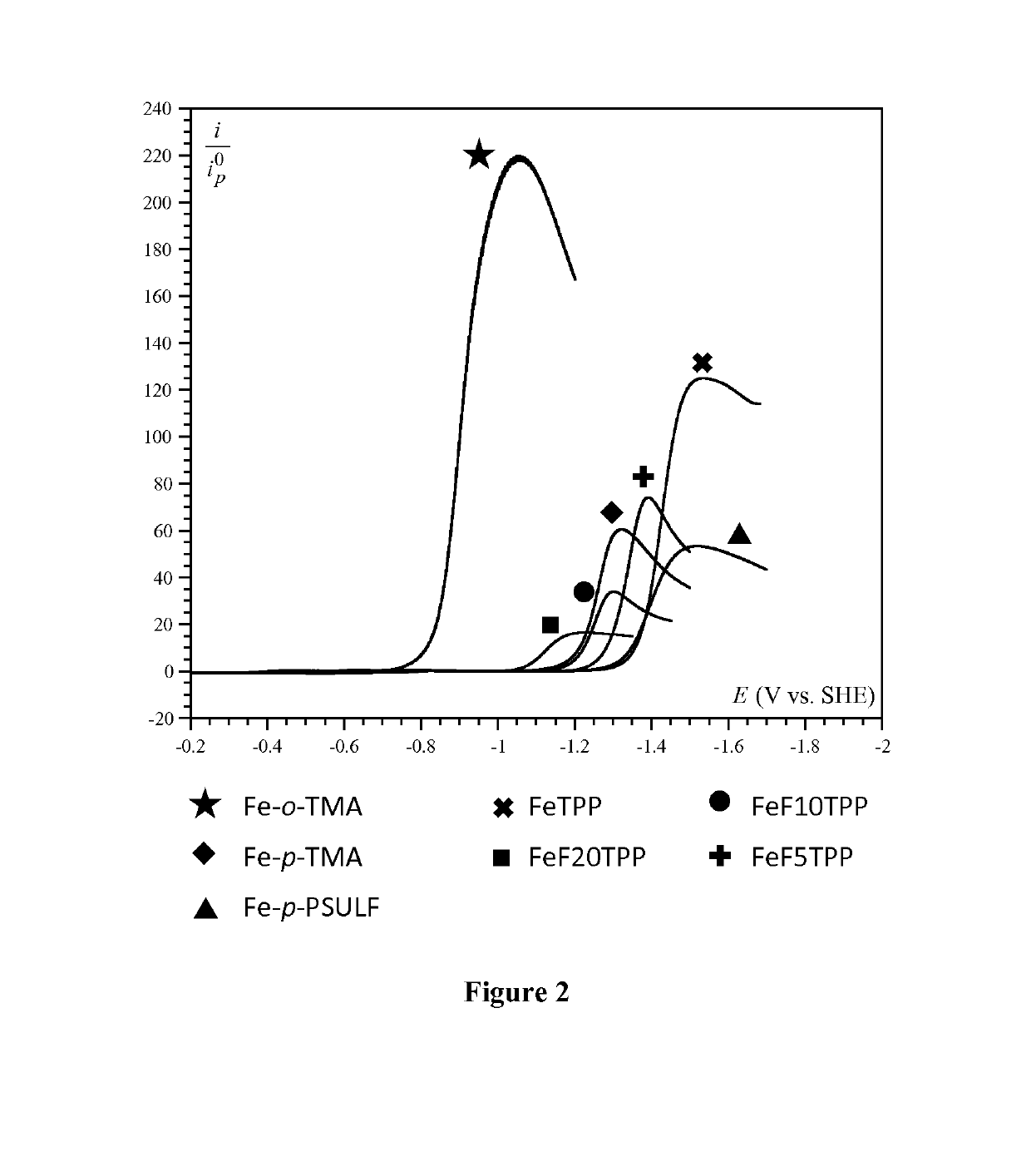
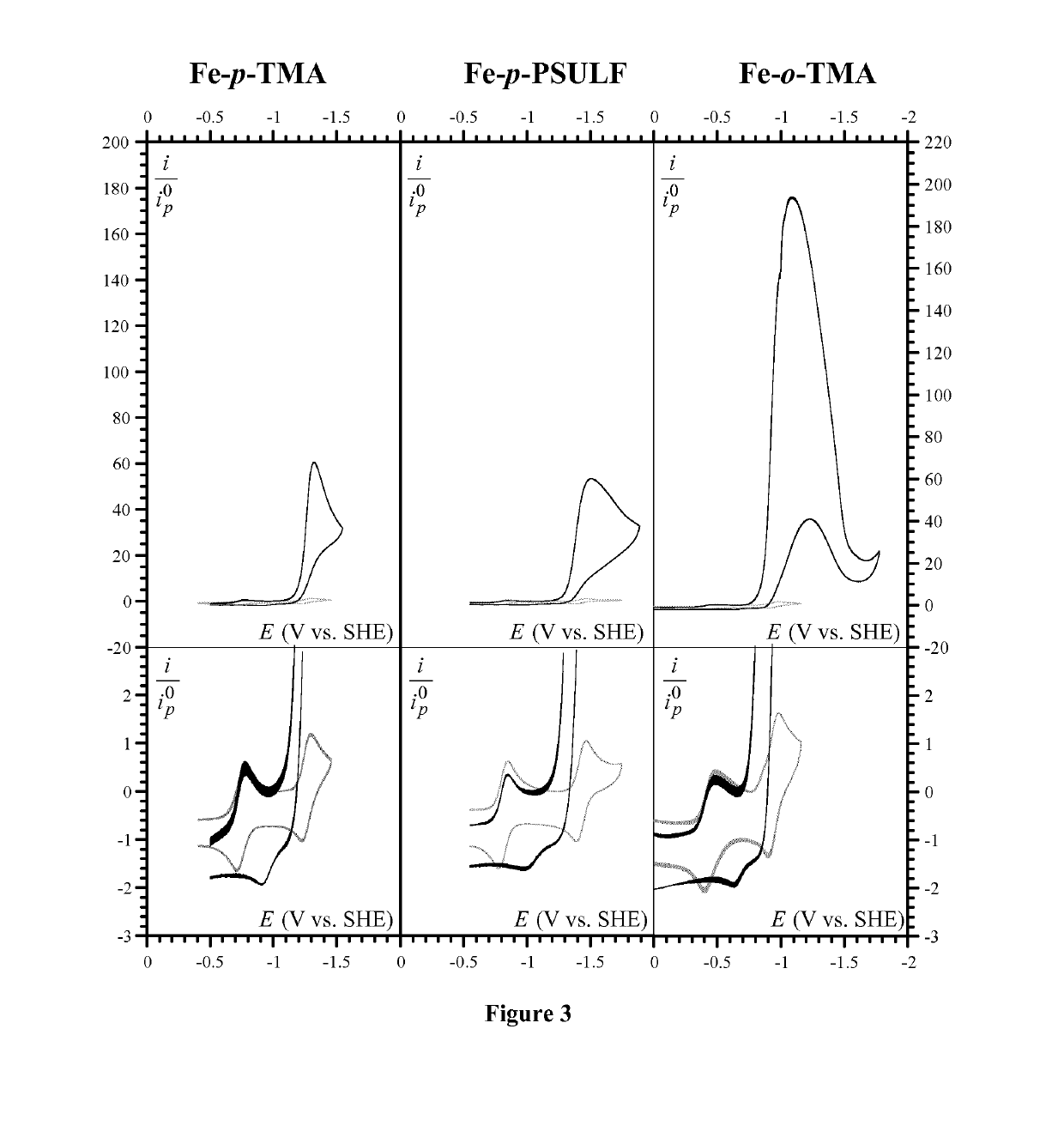
[0167]Iron(III) 5,10,15,20-tetrakis(4′-N,N,N-trimethylanilinium)-porphyrin pentachloride (Fe-p-TMA) was prepared as described elsewhere in Costentin et al. (Proc.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pressure | aaaaa | aaaaa |
| Pressure | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap