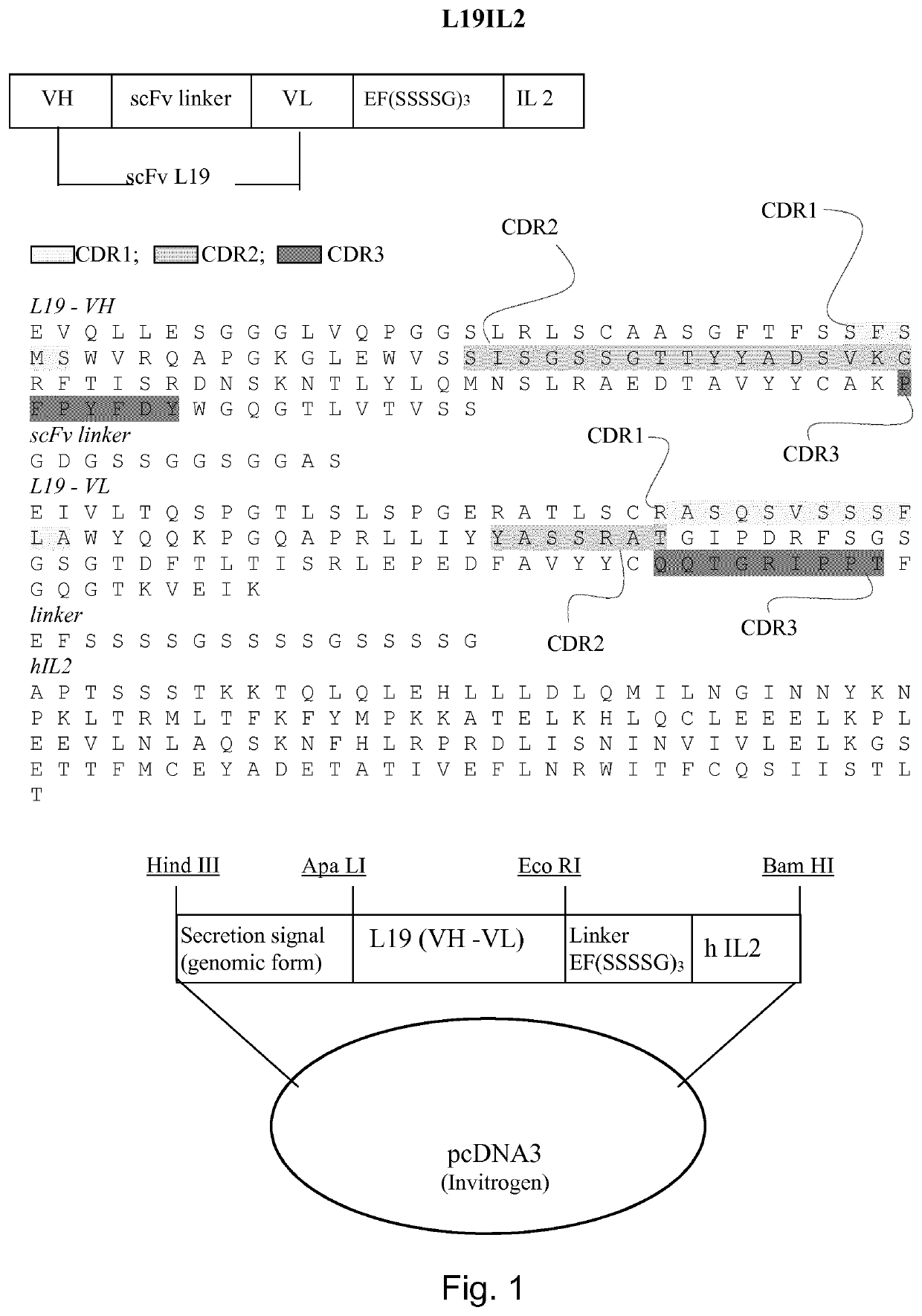
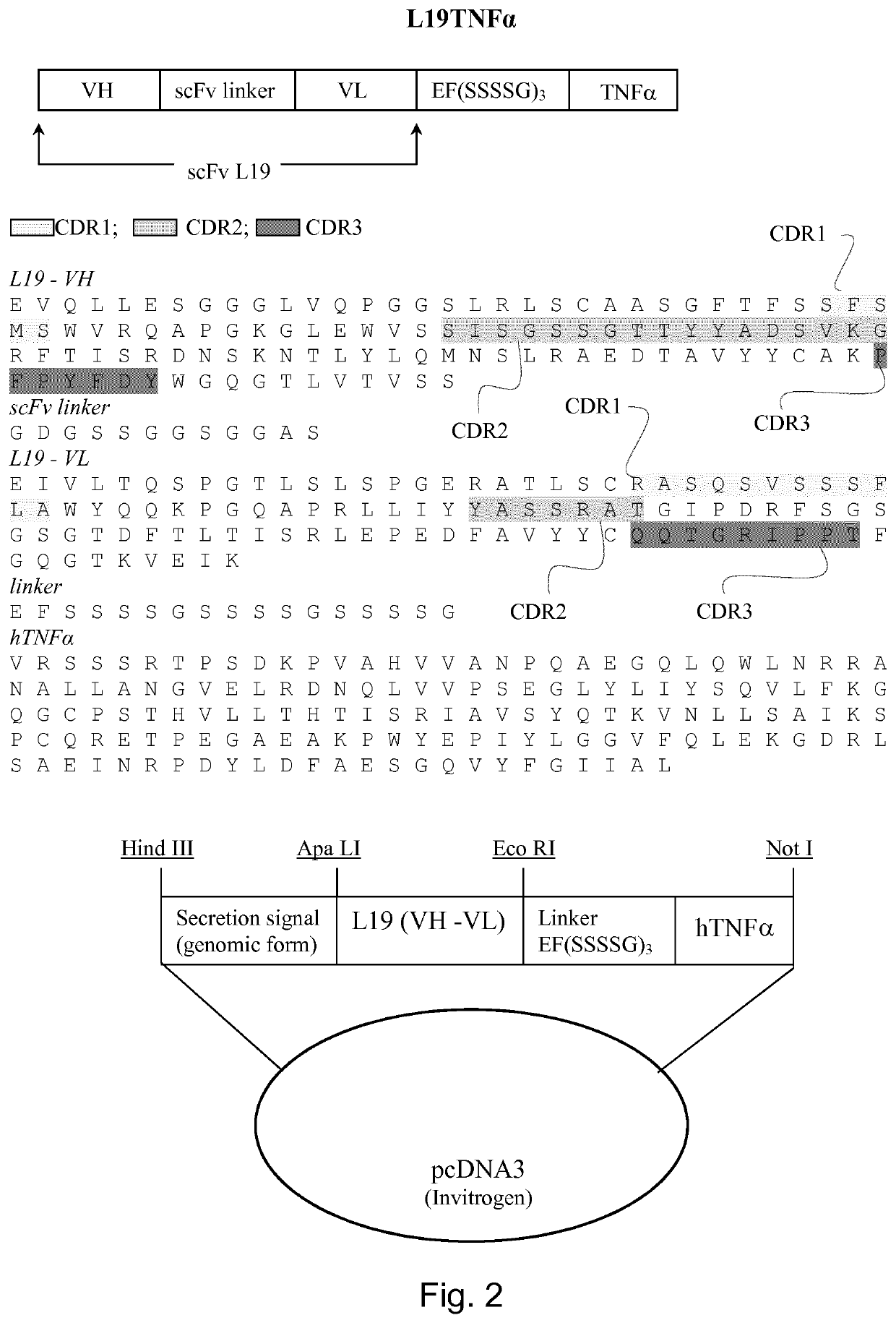
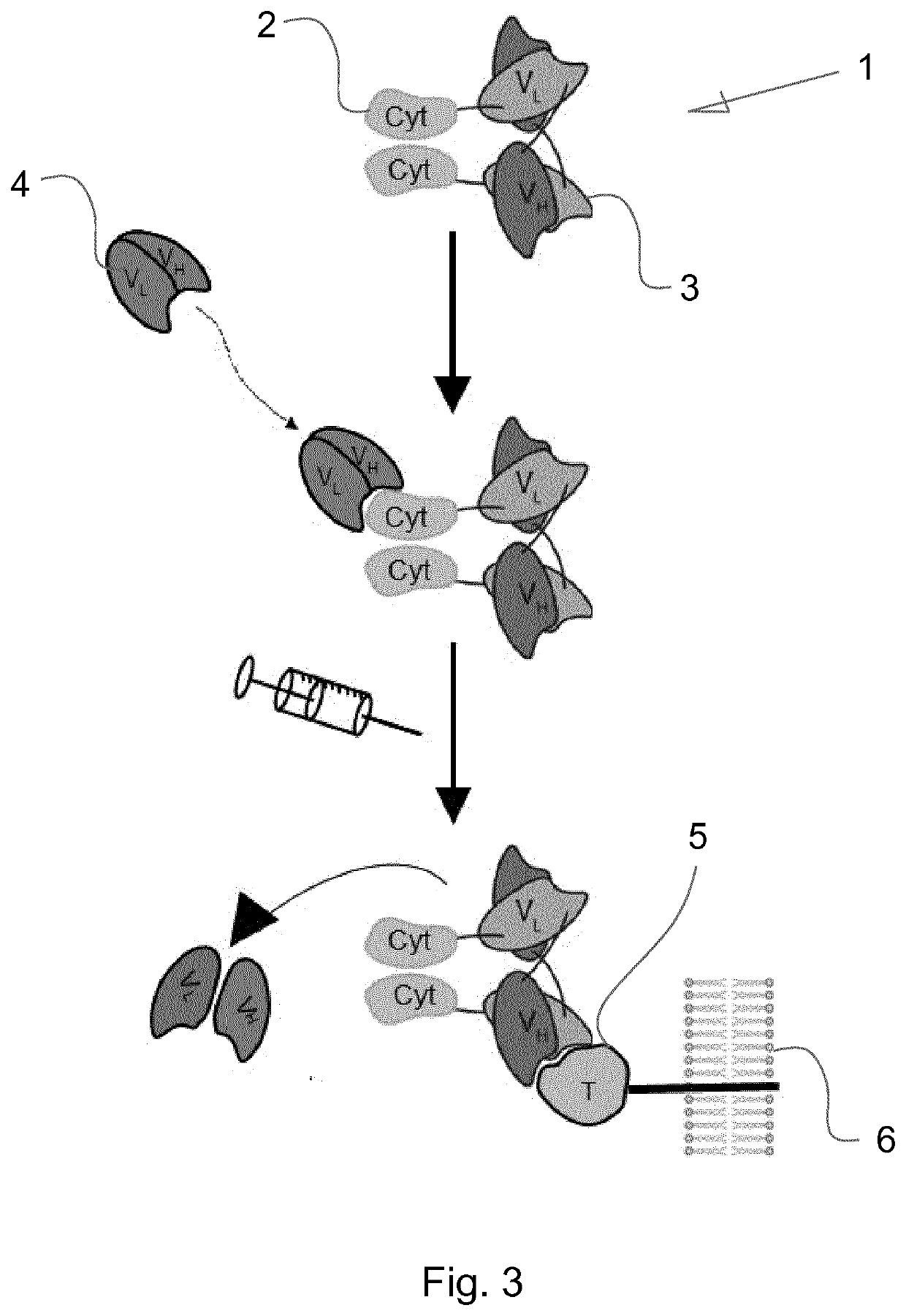
Immunocytokines with progressive activation mechanism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
on of an Anti L19-IL2 Organic Binder
[0237]Through dual-pharmacophore DNA-encoded chemical libraries we discovered two fragments that synergize in the binding to IL2 fused to the immunocytokine L19-IL2. The fragments are reported in FIG. 5A.
[0238]Fragment A was already reported as weak-micromolar binder and inhibitor of IL2 (Leimbacher et al (2012).
[0239]The two fragments were connected using a series of bi-functional scaffolds bearing a proper fluorescent tag for affinity measurements through florescent polarization techniques. The structures of the three compounds tested are reported in FIG. 5B. Affinity constants (KD) for compounds 1-3 measured by florescent polarization are reported in Table 1.
Compound IDKD (nM)119 ± 7222 ± 83 29 ± 12
[0240]Materials and Methods
[0241]General Remarks and Procedures
[0242]Synthetic oligonucleotides were purchased from various commercial suppliers and stored as water solutions at −20° C. Chemical compounds were purchased from various commercial suppliers
example 2
on of Anti-IL2 scFv Antibodies
[0309]Material & Methods
[0310]Antigen Preparation for Antibody Phage Display Selections
[0311]The fusion protein antibody (L19)-interleukin 2 (L19-IL2) was used as antigen to isolate human antibody fragments specific to IL2. L19-IL2 was biotinylated using EZ-Link® NHS-Biotin Reagent (Thermo Scientific) in order to attach 2 biotin moieties per L19-IL2 molecule.
[0312]Antibody Selection Protocol
[0313]Antibody selection was performed essentially as described in Silacci et al (2005). 60 ul of magnetic dynabeads (Invitrogen) were coated with 120 pmol of biotinylated antigen. PHILO1 and PHILO2 libraries were used for antibody selection (WO2010 / 028791). In order to prevent the isolation of binders specific to the L19-antibody portion of the antigen, we added as competitor 10−6M L19 antibody in Sip format (WO2003 / 076469) to the 2% (w / v) skimmed milk / PBS blocking solution (MPBS). L19-IL2-coated dynabeads were incubated with antibody libraries (in 2% MPBS with
example 3
ctivity Experiments
[0329]Material & Methods
[0330]CD1 mice were divided in five groups and received one i.v. injection of the following dosages:
[0331]Group 1: 200 μg L19-IL2 (4.8 nmol)
[0332]Group 2: 200 μg L19-IL2 (4.8 nmol)+200 μg KSF (8 nmol)
[0333]Group 3: 200 μg L19-IL2 (4.8 nmol)+200 μg EKH3 (8 nmol)
[0334]Group 4: 200 μg L19-IL2 (4.8 nmol)+160 μg PLG5 (6.4 nmol)
[0335]Group 5: 200 μg L19-IL2 (4.8 nmol)+LSD5-61 in 1.25% DMSO (2.5 nmol)
[0336]All mice were weighed daily and kept in constant observation for clinical examination. The weight changes of the five groups are reported in FIG. 9.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap