Salivary Gland Cell Sheets and Methods for their Production and Use
a salivary gland and cell sheet technology, applied in the field of salivary gland cell sheet, can solve the problems of limited treatment, slow progress in cured hyposalivation, and low level of structural organization, and achieve the effect of increasing saliva flow ra
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
on and In Vitro Characterization of Mouse Submandibular Gland (SMG) Cell Sheets
[0064]Methods
[0065]Animals
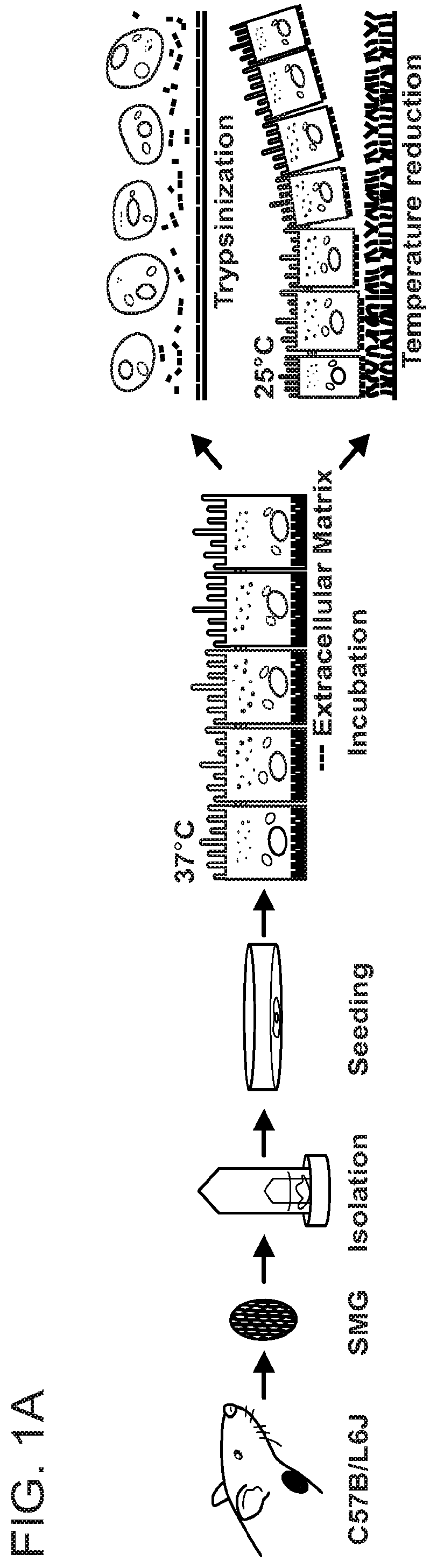
[0066]Female C57BL / 6J mice 6-weeks-old, weighing approximately 17-20 g, were purchased from the Jackson Laboratory (Bar Harbor, Me.). All animal usage, anesthesia, and surgeries were conducted with the approval of the University of Utah Institutional Animal Care and Use Committee, in accordance with their strict guidelines.
[0067]Fresh SMG Cell Isolation
[0068]Mice were euthanized using 80-100 mg / kg Ketamine+5 mg / kg Xylazine followed by abdominal exsanguination. SMG were then removed, cut into small pieces and placed in a 35 ml GentleMACS™ C Tube containing 6.5% tumor dissociation enzyme mixture (Miltenyi Biotec Inc. Auburn, Calif.) in DMEM / F12 (Invitrogen, Carlsbad, Calif.). Subsequently, the tissue was dissociated using a GentleMACS (Miltenyi Biotec Inc) and incubated in a shaking water bath at 37° C. for 30 min. After three such steps and two intervening incubations, SMG ce
example 2
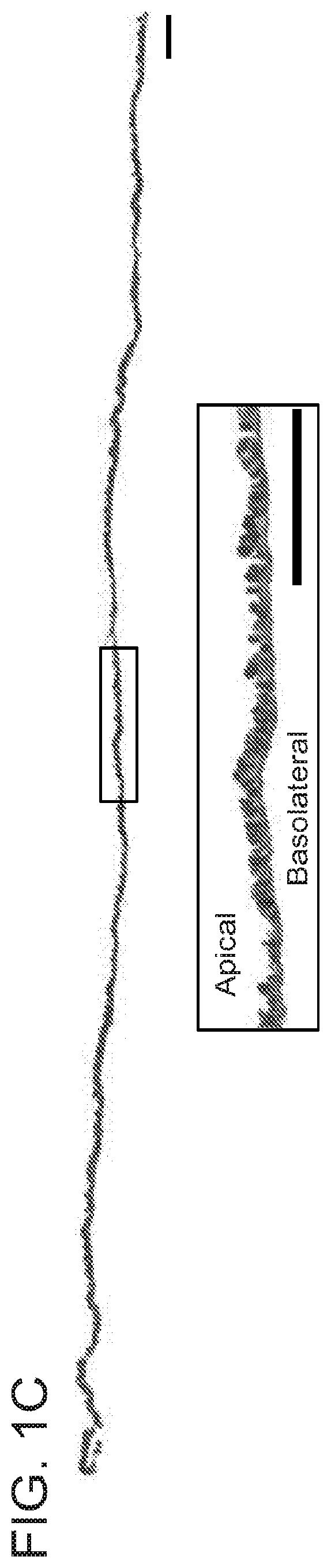
haracterization of Mouse Submandibular Gland (SMG) Cell Sheets
[0087]Methods
[0088]Animal Model
[0089]The wounded SMG model was created following a method reported previously (Nam et al., J Dent Res. 2017; 96:798-806; Nam et al., PLoS ONE. 2017; 12:e0187069). Briefly, C57BL / 6J mice were anesthetized with 3% isoflurane with an oxygen flow rate set at 2.0 L / min, SMGs were exposed and surgical wounds created using a 3 mm diameter biopsy punch. The treatment groups were as follows: (1) wounded and treated with a single layer cell sheet (experimental group SC), (2) wounded and treated with a double layer cell sheet (experimental group DC), (3) untreated wounded control or (4) unwounded (sham surgery controls). After surgery, the skin incision was sutured, and post-surgical studies were performed at day 8 or 20. For these purposes, SMG were dissected and processed for histological analysis and saliva secretion studies as described below.
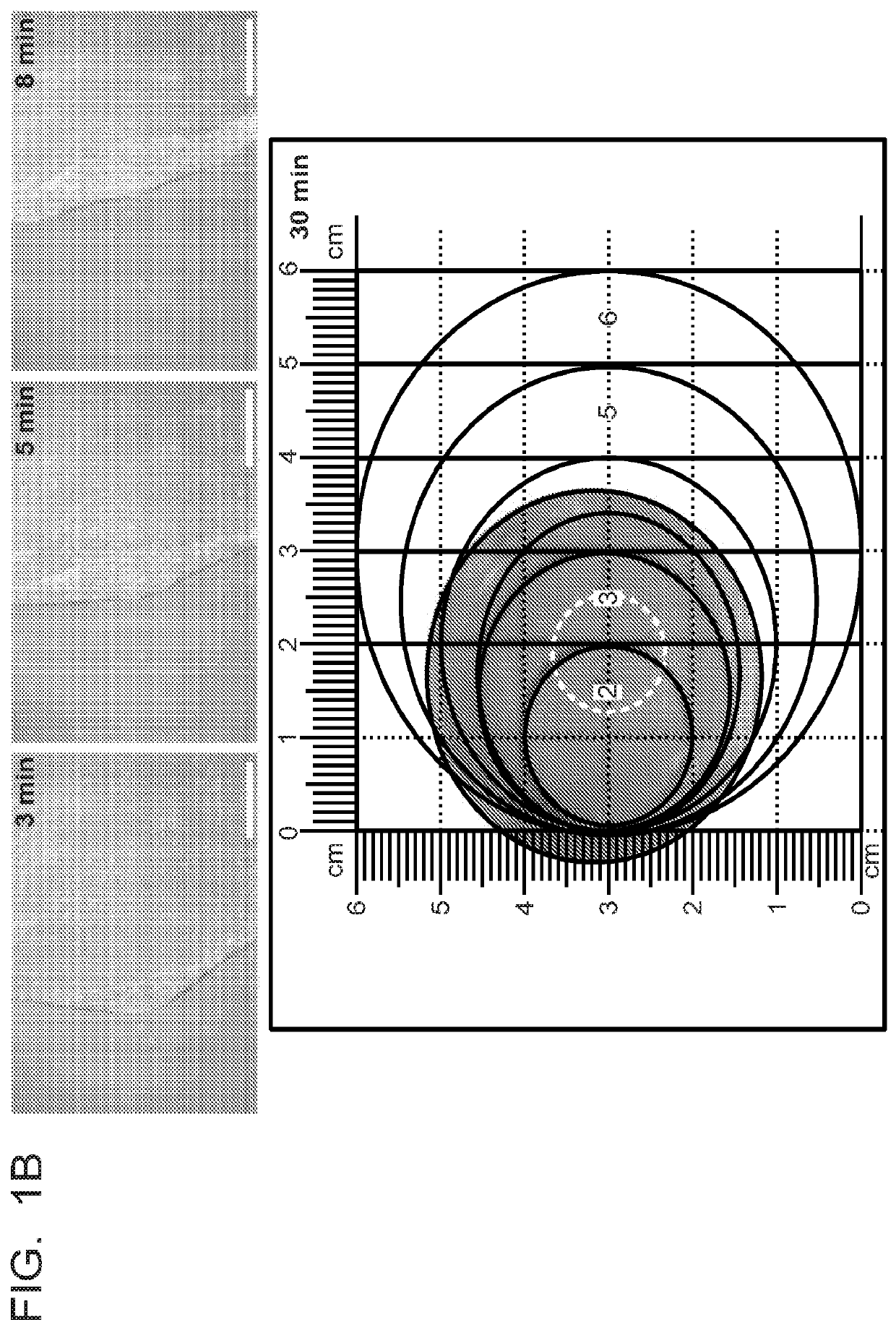
[0090]Saliva Flow Rate Measurements
[0091]To collect st
example 3
on of Human Submandibular Gland (SMG) Cell Sheets
[0103]To isolate human SMG cells for preparation of cell sheets, human SMG tissue (about 100 mg) was cut into small pieces and placed in a 35 ml GentleMACS™ C Tube containing 6.5% tumor dissociation enzyme mixture (Miltenyi Biotec Inc. Auburn, Calif.) in DMEM / F12 (Invitrogen, Carlsbad, Calif.). The tissue was dissociated using a GentleMACS dissociator and incubated in a shaking water bath at 37° C. for 30 min. After three dissociation steps and two incubation steps, SMG cells were centrifuged at 150×g for 5 min at 4° C. and the medium was removed. The cells were then resuspended in 5 ml DMEM / F12 complete medium containing the following: 2.5% FBS, 2 nM triiodothyronine, 0.1 μM retinoic acid, 0.4 μg / ml hydrocortisone, 80 ng / ml EGF, 5 ng / ml sodium selenite, 5 mM glutamine, 5 μg / ml insulin and 5 μg / ml transferrin, and passed through 70 μm and 40 μm strainers (Thermo Fisher Scientific, Waltham, Mass.). Then, 1.5×106 cells were s
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap