Polypeptides having opioid receptor activity, nucleic acids coding therefor and uses thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example
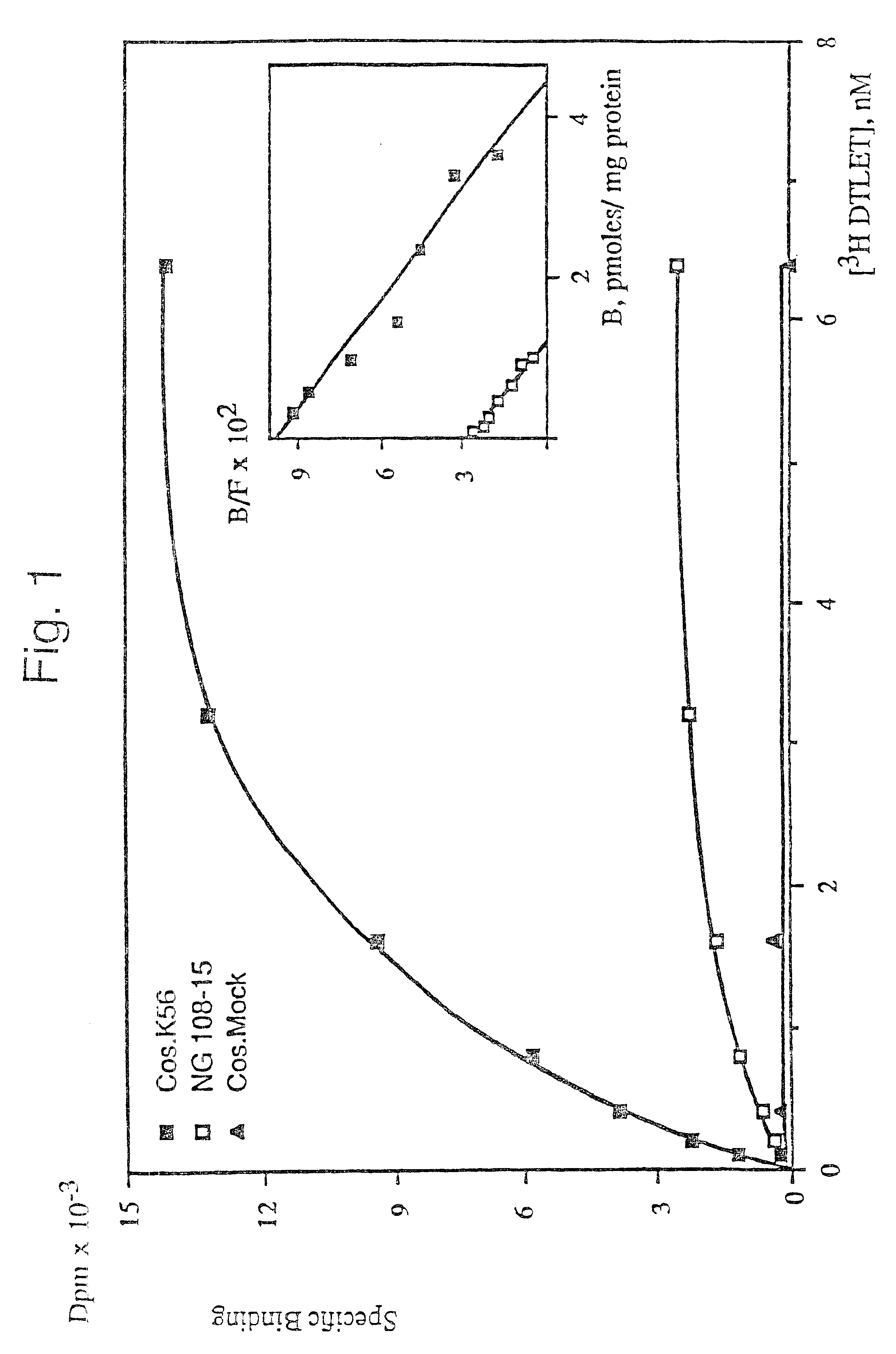
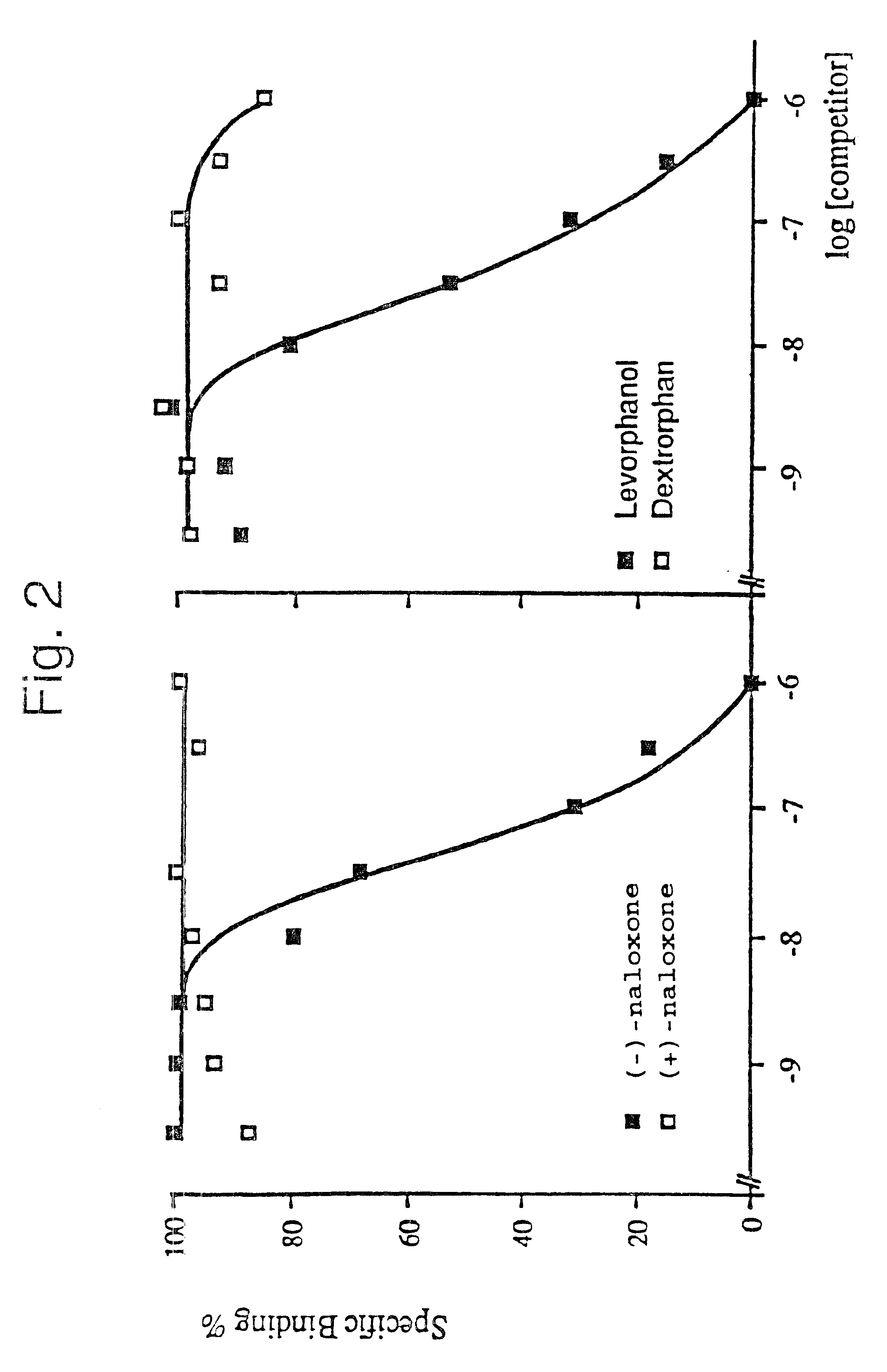
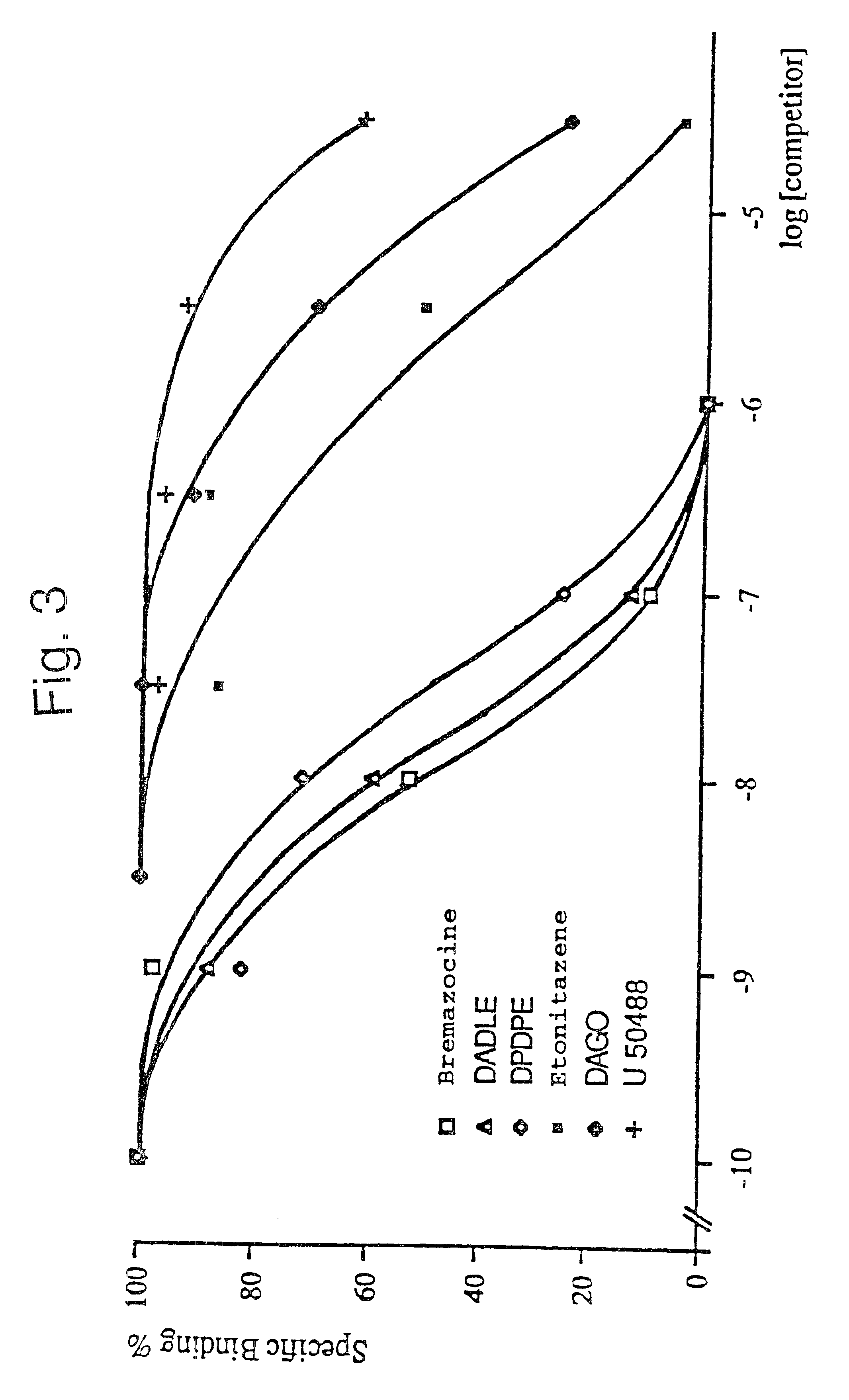
The K56 plasmid isolated in Example 1 was used to transfect Cos-1 cells. The membranes of the transfected cells which were obtained were then prepared and tested for their ability to bind certain labelled opioid ligands.
The caesium chloride-purified plasmid K56 (plasmid pCDM8 which contains the insert of approximately 2.2 kb encoding the opioid receptor) was used to transfect Cos-1 cells (on a 10 cm plate) using the DEAE dextran technique. The control consists of cells which are transfected with the pCDM8 plasmid.
72 hours after transfection, the recombinant cells are harvested and the membranes are prepared in the following manner: the cell pellets are taken up, at 4.degree. C., in 60 ml of 50 mM Tris-HCl, pH 7.4; 10 mM EDTA buffer, then homogenized and centrifuged at 1100 g for 10 minutes. The pellet is subsequently taken up for a second time in 30 ml of the same buffer, and homogenized and centrifuged. The 2 supernatants are combined and centrifuged at 110,000 g for 15 minutes. The m
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap