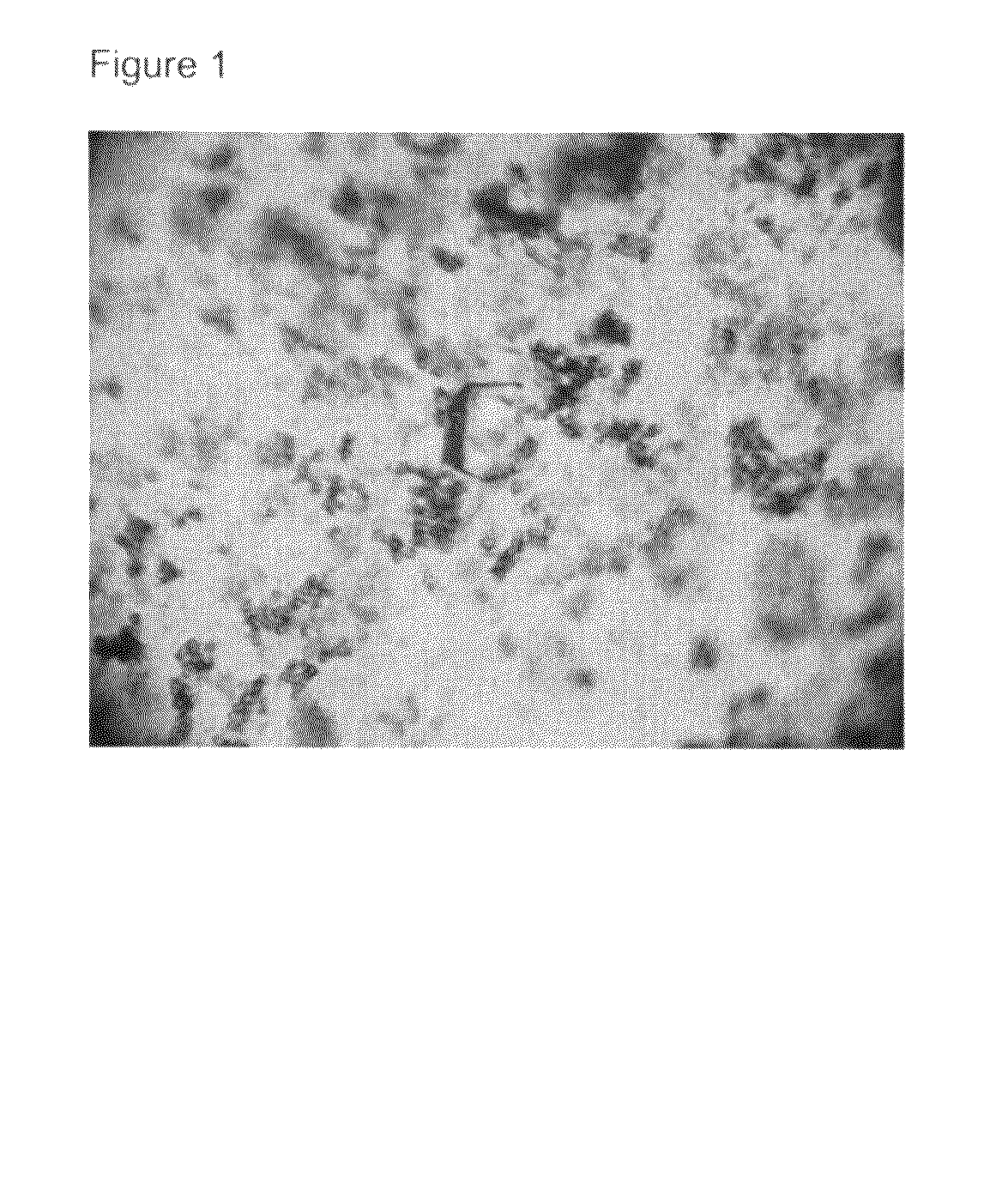
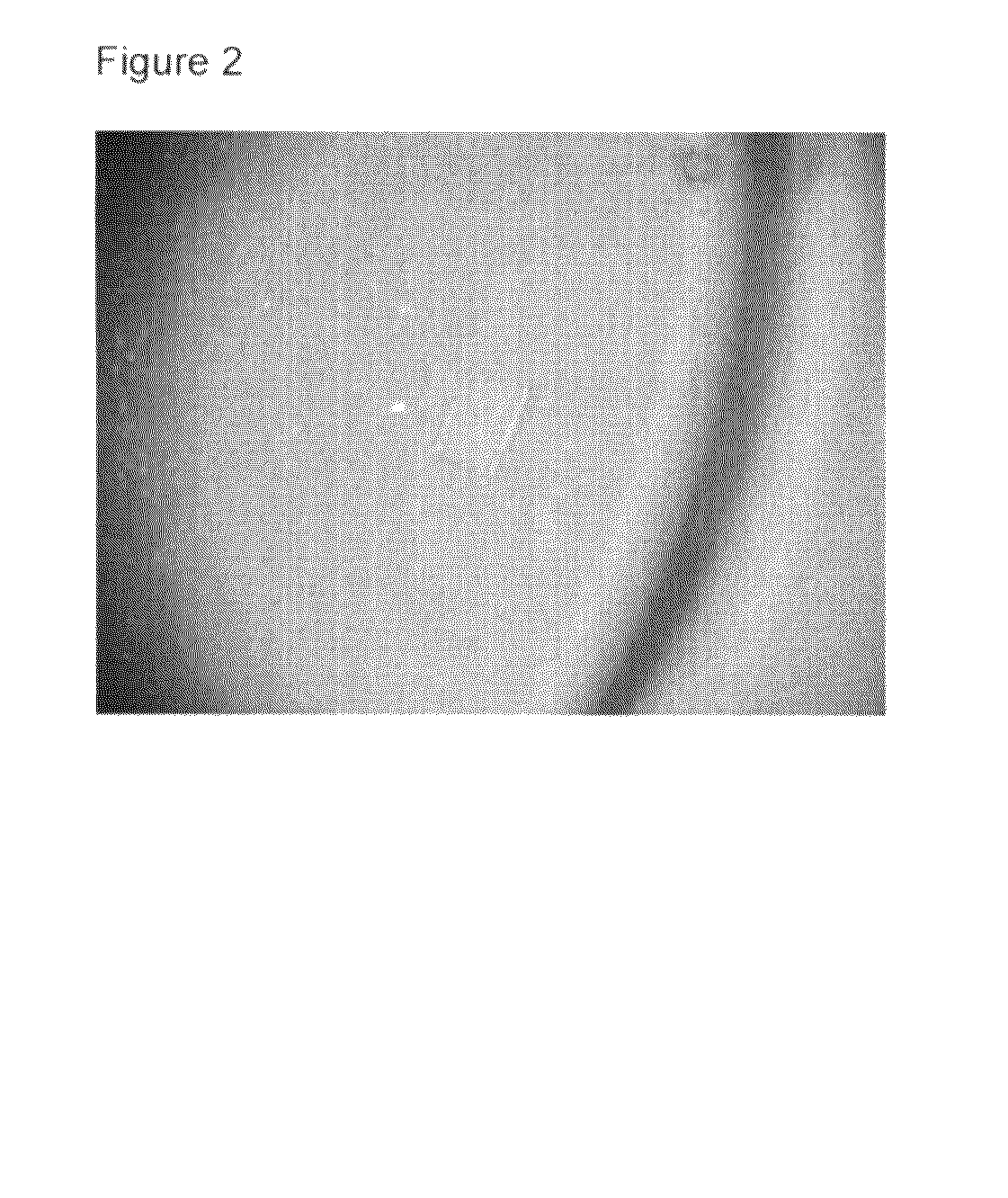
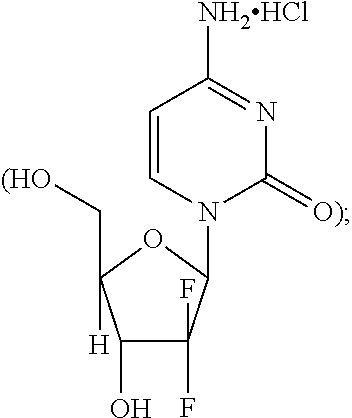
Crystal of human interferon alpha 2B in complex with zinc
a technology of interferon and complexes, applied in the field of interferon polypeptide compositions, can solve the problems of poor patient compliance, limited administration modes of macromolecules, and difficulty in transdermal or oral delivery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation and Purification of Interferon Alpha-2b Dimers Coordinated with Zinc
[0112]The interferon alfa-2 employed was recombinant human interferon alfa-2b expressed in E. coli as described in Weissmann, et al. Science, 209, 1342 (1980). The cells were cultured, harvested and extracted as previously reported in Leibowitz, P. et al (1982) U.S. Pat. No. 4,315,852. Briefly, the extraction involved acidifying the suspension of interferon-containing bacterial cells, removing the suspension liquid from the cells, preparing a second suspension of the acidified cells and neutralizing the second suspension, then separating the interferon containing liquid from the suspended cells, and extracting the interferon from the liquid. This extraction method efficiently released the interferon from the cells, upon neutralization of the suspension of acidified cells, without the need for mechanical or enzymatic disruption of the cell surface. The resulting extracts were purified by a combination of con
example 2
Crystallization Conditions for Flight Experiments
[0113]Pre-Crystallization Processing (Flight).
[0114]All operations were performed under clean room conditions at 4° C. 109 mg of lyophilized interferon alpha 2b amorphous solid as described in example 1, was dissolved in 11 ml of 30 mM sodium acetate (Fluka BioChemika Buchs, AG; sodium acetate anhydrous cat #71183), pH 6.03 (1M Sodium chloride (Fluka BioChemika Buchs, AG; sodium chloride anhydrous cat #71376) stock solution was added drop wise to a conductivity reading of 5 milli-siemens) at 4° C. The resulting solution was dialyzed in a Spectrum (Rancho Dominguez, Ca, USA; Spectra / por RC float-a-lyzer cat #23510; 10 thousand molecular weight cutoff) versus a dialyzate 200 mL of 30 mM sodium acetate, pH 6.03(1 M Sodium chloride stock solution was added drop wise to a conductivity reading of 5 milli-siemens). The dialysis was continued with a fresh dialyzate (200 ml) for 18 hours at 4° C. The resulting solution was filtered using a 0.22
example 3
Ground Crystallization Experiments
[0121]Pre-Crystallization Processing (Ground).
[0122]All operations were performed under clean room conditions at 4° C. 109 mg of lyophilized interferon alpha 2b amorphous solid as described in example 1 was dissolved in 11 ml of 30 mM sodium acetate, pH 6.03 (Fluka BioChemika, Buchs, AG; sodium acetate anhydrous, cat #71183), 1M Sodium chloride (Fluka BioChemika Buchs, AG; sodium chloride anhydrous cat #71376) stock solution was added drop wise to a conductivity reading of 5 milli-siemens) at 4° C. The resulting solution was dialyzed in a Spectrum (Rancho Dominguez, Ca, USA; Spectra / por RC float-a-lyzer cat #235105; 10 thousand molecular weight cutoff) versus a dialyzate 200 ml of 30 mM sodium acetate, pH 6.03 (1 M Sodium chloride stock solution was added drop wise to a conductivity reading of 5 milli-siemens). The dialysis was continued with a fresh dialyzate (200 ml) for 18 hours at 4° C. The resulting solution was filtered using a 0.22 micron 50 m
PUM
| Property | Measurement | Unit |
|---|---|---|
| Digital information | aaaaa | aaaaa |
| Particle diameter | aaaaa | aaaaa |
| Particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap