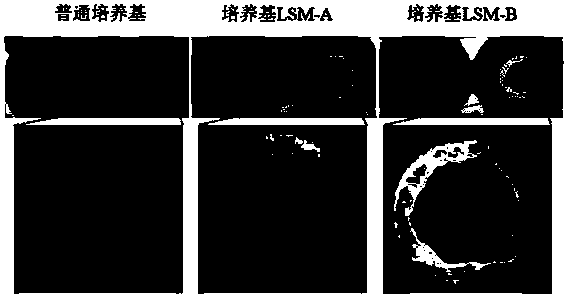
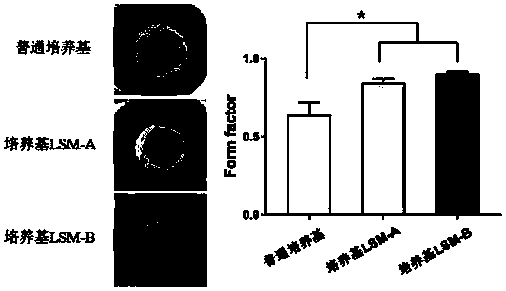
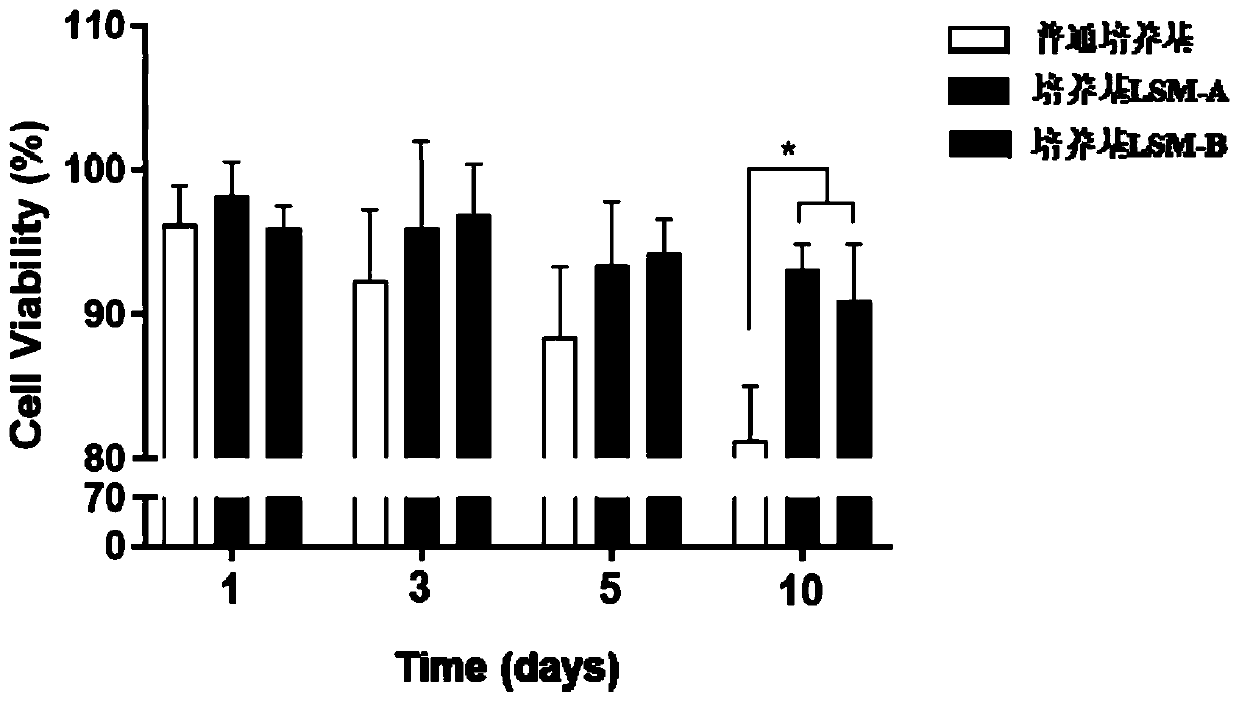
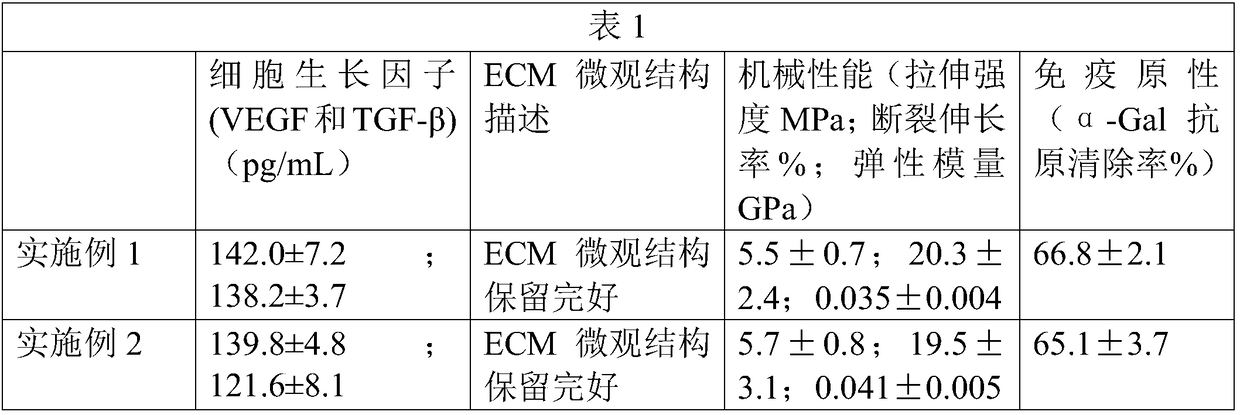
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
6 results about "Cell growth" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The term cell growth is used in the contexts of biological cell development and cell division (reproduction). When used in the context of cell development, the term refers to increase in cytoplasmic and organelle volume (G1 phase), as well as increase in genetic material (G2 phase) following the replication during S phase. This is not to be confused with growth in the context of cell division, referred to as proliferation, where a cell, known as the "mother cell", grows and divides to produce two "daughter cells" (M phase).
Culture medium for hepatoma organoid cell sphere culture
ActiveCN110878286AHigh activityLong-term stable aggregationCulture processCell culture active agentsNutritionPancreatic hormone
Owner:江苏信安佳医疗科技有限公司
Oral cavity defect repair membrane and preparation method thereof
PendingCN109260518AHigh biosecurityLow immunogenicityTissue regenerationProsthesisDefect repairAcellular matrix
Owner:广州聚明生物科技有限公司
Wild ganoderma product for resisting cancer and tumor and removing liver toxin and preparation technology
InactiveCN108968031AGood anti-corrosion and fresh-keeping effectStrengthen cancer treatmentFruit and vegetables preservationFood ingredient functionsDiseaseNasopharyngeal carcinoma
Owner:深圳玺越建材科技有限公司
Antibacterial dressings for promoting healing of skin wounds and preparation method thereof
InactiveCN108404195AGood compatibilityImprove antibacterial propertiesAbsorbent padsBandagesPolyesterFreeze-drying
Owner:代清燕
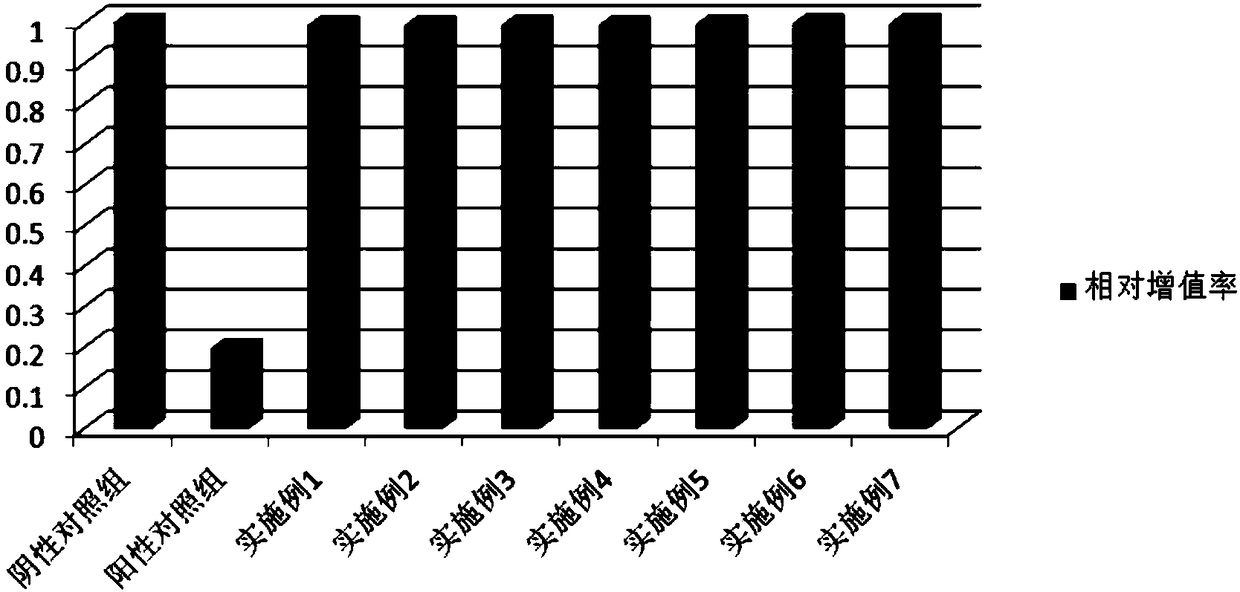
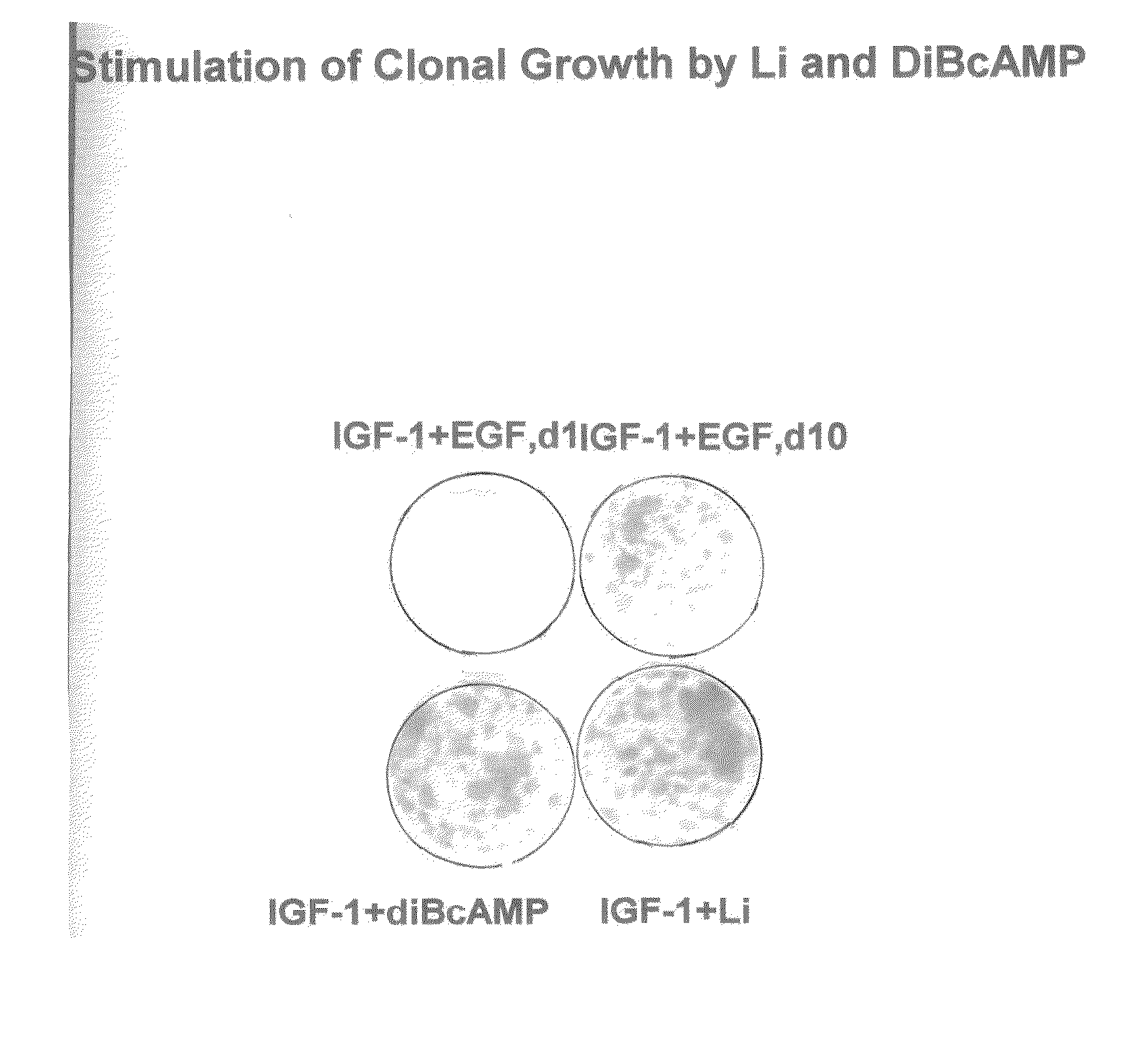
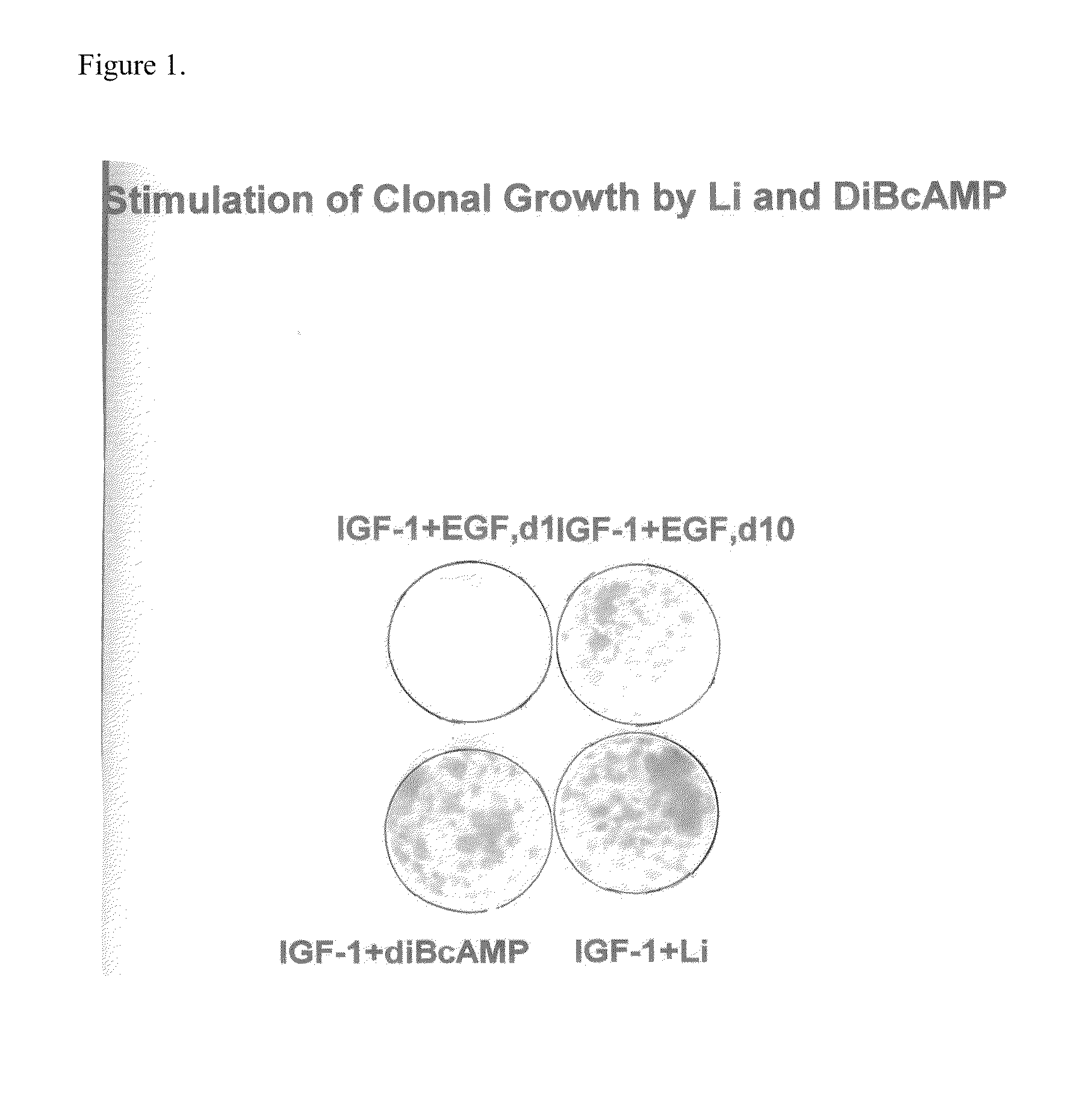
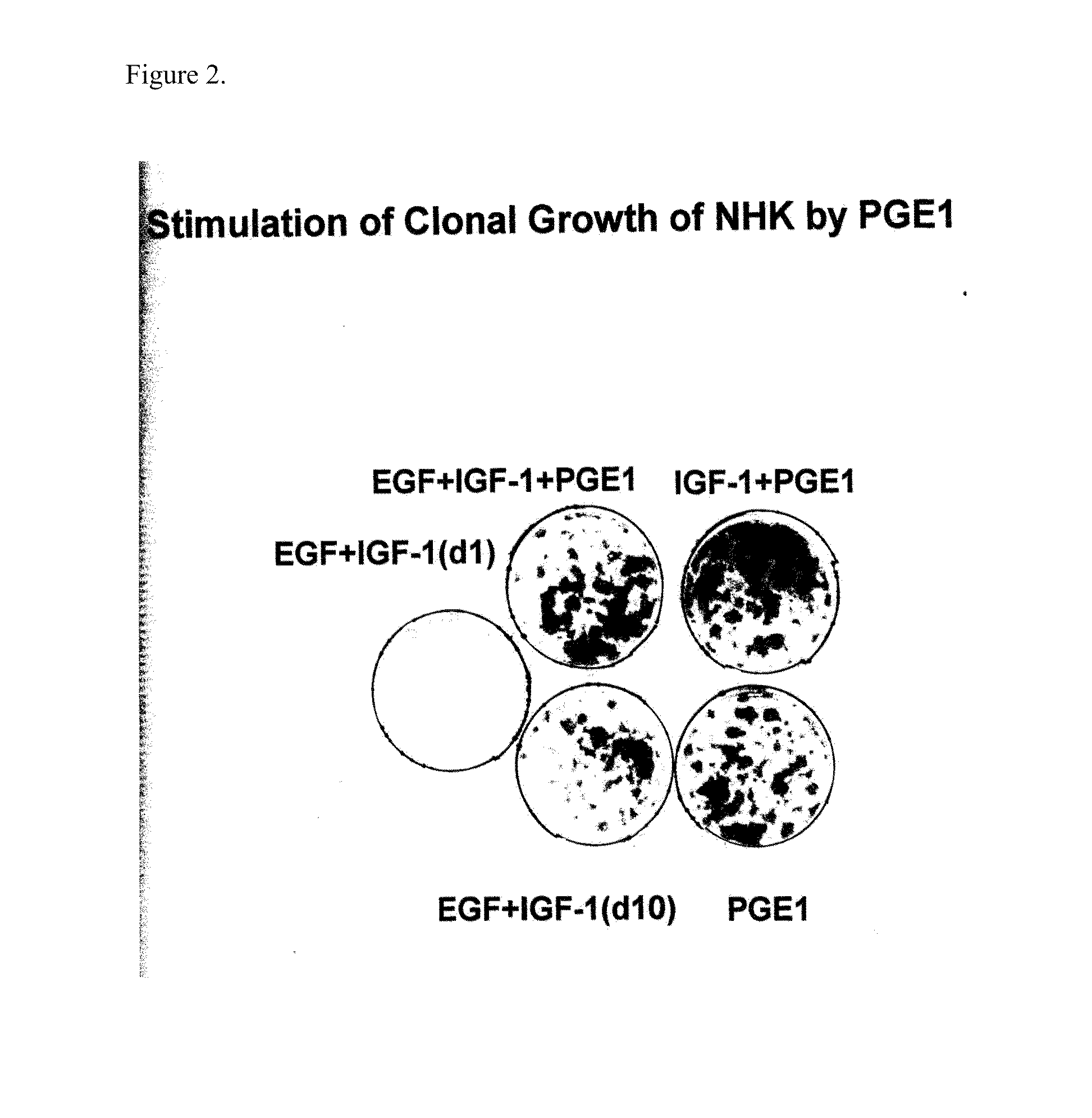
Enhancement of epidermal cell growth by non-protein growth factors
InactiveUS20110300628A1Good effectEpidermal cells/skin cellsCulture processInsulin-like growth factorIsoprostaglandin E1
Owner:WILLE JR JOHN JACOB
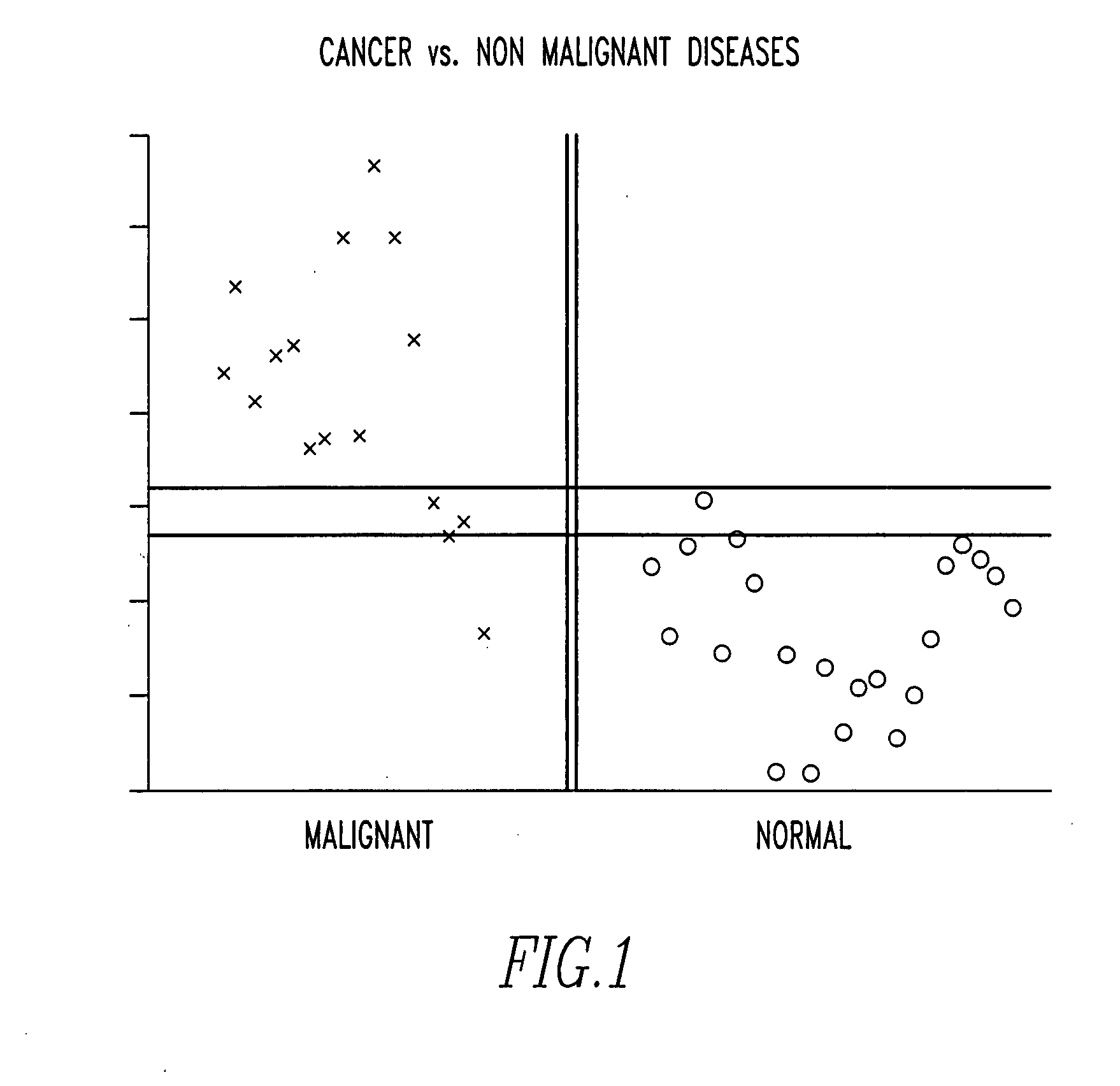
Detection and treatment of cancer
InactiveUS20070237760A1Biological material analysisImmunoglobulins against cell receptors/antigens/surface-determinantsCancer researchCell growth
Owner:CAMOFI MASTER LDC +1
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap