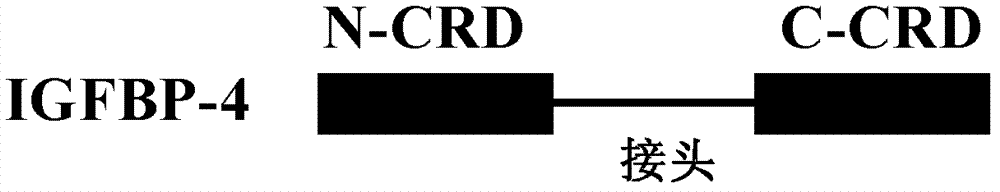
Protein and pharmaceutical composition for treating ischemic diseases
An ischemic disease and drug technology, applied in the fields of bioengineering and biomedicine, which can solve the problems of limited number of mature cardiomyocytes, inability to repair diseased myocardium, and low number of organ donations.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Preparation of IGFBP-4 protein
[0086] 1. Cloning the IGFBP-4 gene:
[0087] cDNA was obtained by reverse transcription of human mRNA extracted by conventional methods. Using the cDNA as a template, using primers corresponding to both sides of the ORF frame of the IGFBP-4 gene (SEQ ID NO.: 1), the amplification product of human IGFBP-4 was obtained by RT-PCR amplification, and the length of the product was consistent with the predicted value .
[0088] 2. Construction of IGFBP-4 eukaryotic expression vector:
[0089] The PCR amplification product obtained above was subcloned by TA into the pcDNA3.1-TOPO-6XHis eukaryotic cell expression vector (purchased from Invitrogen).
[0090] 3. Transfection:
[0091] The host cells were transfected into HEK293 cells by conventional methods, and cultured under the condition of 500 μg / ml G418 to obtain multiple growing HEK293 cell lines, namely transformants.
[0092] Select the transformant with high expression level and cultiv
Embodiment 2
[0096] Preparation of Animal Lower Limb Ischemia-Ischemia Model
[0097] 1. Model Establishment and Specimen Collection
[0098] Ten 8-week-old male C57BL / 6 mice (The Jackson Laboratory, USA) were randomly divided into an experimental group and a control group, with 5 mice in each group. Directly inject IGFBP-4 (5μg / 50μl) into the ischemic lower limbs at several points, and inject the same volume of normal saline to the mice in the control group. Laser Doppler imaging shows ischemic parts in blue and bloody parts in red.
[0099] 2. When the mice were 12 weeks old, the left femoral artery was ligated and resected to establish the mouse lower limb ischemia model. Before operation, immediately after operation, and 7, 14, 21, 28 days after operation, the blood perfusion of lower limbs of mice was monitored by laser Doppler ultrasound scanner.
Embodiment 3
[0101] Animal lower limb ischemia model treated with drugs
[0102] After the mouse lower limb ischemia model was established (embodiment 2), the blood perfusion situation of the mouse lower limbs was monitored with a laser Doppler ultrasonic scanner; the mouse tail vein was utilized to inject recombinant IGFBP-4 protein (5 μg / 25g) or Placebo (PBS) was used as a control; after 6 days, the blood perfusion of lower limbs of mice was also monitored by laser Doppler ultrasound scanner.
[0103] Observation and evaluation of therapeutic effects: the results showed that the lower limb ischemia of mice in the one-time injection of recombinant IGFBP-4 protein group was significantly improved, Figure 4 shows the results of IGFBP-4 protein for animal treatment, wherein, Figure 4 A is the direct observation result of anatomy; Figure 4 B is the infrared image of blood vessels on day 0 after treatment; Figure 4 C is the vascular infrared imaging image 6 days after treatment, the left
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap