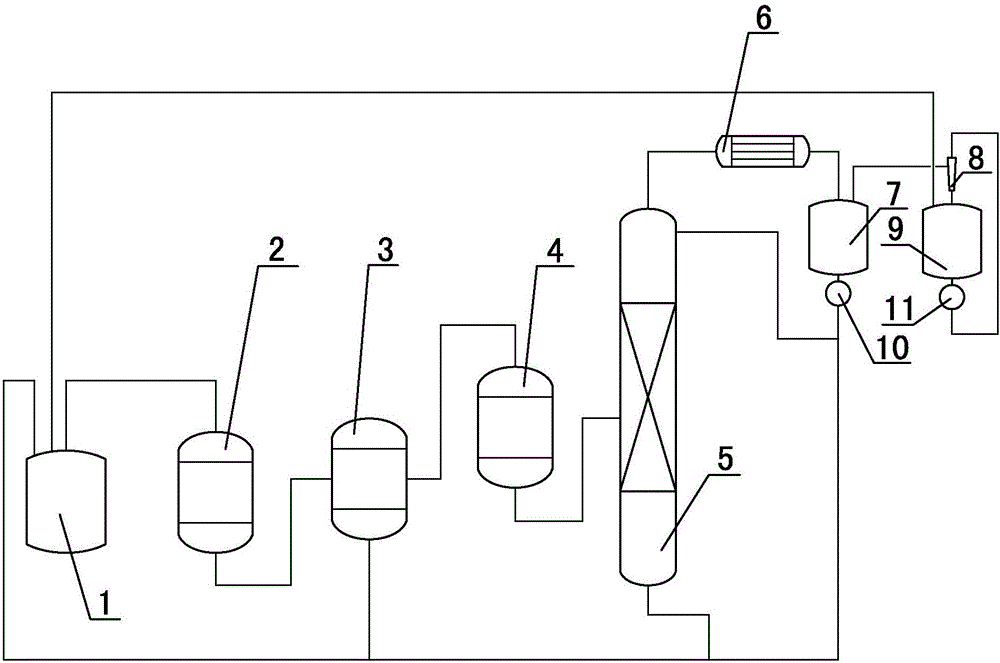
Production process of sulfoxide chloride
A technology of thionyl chloride and production process, applied in the field of production technology of thionyl chloride, can solve the problems of increasing the production load of a rectifying tower, reducing the safety performance of the device, and being unable to condense tail gas, thereby reducing energy consumption and ensuring conversion. Efficiency and load reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0019] Example 1
[0020] (1) The synthesis gas produced by the reaction of sulfur and chlorine in the synthesis kettle enters the reactor together with sulfur dioxide gas, and the crude thionyl chloride generated by the reaction in the reactor is directly decolorized in the gaseous form through the sulfur tank and then enters the decomposer, the sulfur tank The bottom liquid phase is recycled to the synthesis kettle;
[0021] (2) The crude gas phase thionyl chloride from the decomposer enters the rectification tower, and the heavy components produced at the bottom of the rectification tower are recycled back to the synthesis kettle. The gas phase at the top of the rectification tower enters the reflux tank after condensation, and part of the liquid in the reflux tank The phase is pumped by the first machine to the rectification tower to reflux, and the remaining liquid phase in the reflux tank is recovered and reused in the synthesis kettle; the volume ratio of part of the liquid pha
Example Embodiment
[0023] Example 2
[0024] (1) The synthesis gas produced by the reaction of sulfur and chlorine in the synthesis kettle enters the reactor together with sulfur dioxide gas, and the crude thionyl chloride generated by the reaction in the reactor is directly decolorized in the gaseous form through the sulfur tank and then enters the decomposer, the sulfur tank The bottom liquid phase is recycled to the synthesis kettle;
[0025] (2) The crude gas phase thionyl chloride from the decomposer enters the rectification tower, and the heavy components produced at the bottom of the rectification tower are recycled back to the synthesis kettle. The gas phase at the top of the rectification tower enters the reflux tank after condensation, and part of the liquid in the reflux tank The phase is pumped by the first machine to the rectification tower for reflux, and the remaining liquid phase in the reflux tank is recovered and reused in the synthesis kettle; the volume ratio of part of the liquid ph
Example Embodiment
[0027] Example 3
[0028] (1) The synthesis gas produced by the reaction of sulfur and chlorine in the synthesis kettle enters the reactor together with sulfur dioxide gas, and the crude thionyl chloride generated by the reaction in the reactor is directly decolorized in the gaseous form through the sulfur tank and then enters the decomposer, the sulfur tank The bottom liquid phase is recycled to the synthesis kettle;
[0029] (2) The crude gas phase thionyl chloride from the decomposer enters the rectification tower, and the heavy components produced at the bottom of the rectification tower are recycled back to the synthesis kettle. The gas phase at the top of the rectification tower enters the reflux tank after condensation, and part of the liquid in the reflux tank The phase is pumped by the first machine to the rectification tower to reflux, and the remaining liquid phase in the reflux tank is extracted and reused in the synthesis kettle; the volume ratio of part of the liquid pha
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap