Preparation and application of fusidic acid chemically modified compound
A technology of geoacid chemistry and fusidic acid, applied in the field of preparation and application of chemical modifiers of fusidic acid, can solve problems such as shortage and less anti-inflammatory drugs, achieve high synthesis yield, simple preparation method, and wide application range wide effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of dihydrofusidic acid:
[0034]
[0035] First, weigh 1.0g (1.94mmol) of fusidic acid into a 100mL dry round-bottomed flask containing magnets, then add 50mL of dry ethanol into the flask, and stir magnetically at room temperature until it dissolves completely Finally, 0.10 g (0.19 mmol) of 5% Pt / C was added, and finally, the reaction was filled with hydrogen for 3 h. The end point of the reaction was detected by TLC (developing agent: petroleum ether: ethyl acetate = 2:1, chromogen: methanol: acetic acid: concentrated sulfuric acid: anisaldehyde (volume ratio) = 85:10:5:0.5), and dihydrogen R of fusidic acid f =0.17 (raw material R f is 0.14). The target compound was obtained as a white solid by steps of filtration, extraction and drying, and the yield was 100%. and by NMR 1 H NMR, 13 C NMR analysis, elemental analysis, high-resolution mass spectrometry, etc. for structural identification.
Embodiment 2
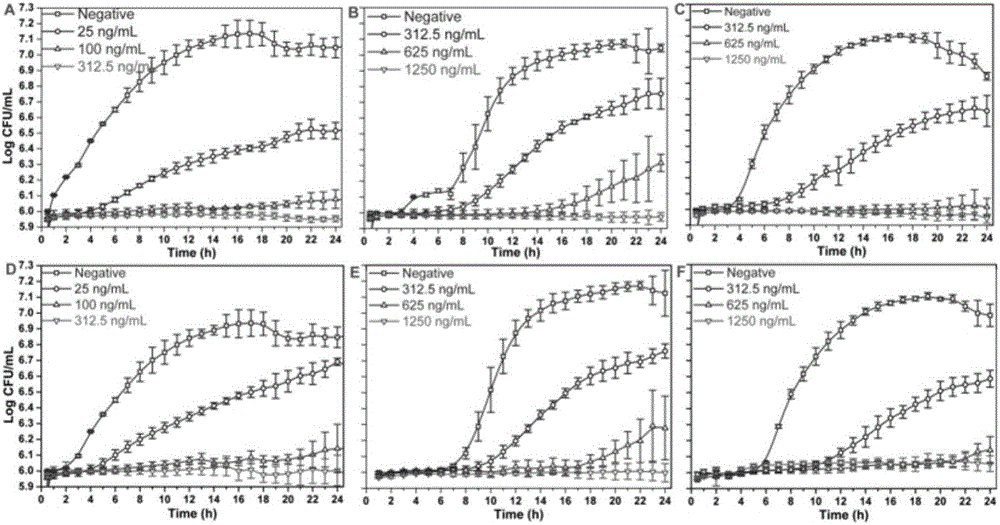
[0037] Bacterial inhibition zone test: The inhibition zone test of fusidic acid and dihydrofusidic acid is mainly determined by the agar disk diffusion method. First, the appropriate bacterial concentration (1×10 7 ~1×108 CFU / mL), the bacterial suspension was inoculated into a 120mm glass culture product containing agar medium, and a filter paper sheet with a diameter of 6mm was attached at the same time. Each agar culture dish had three groups of samples, including two different concentrations of test samples and a negative control group. Then, use a 10 μL pipette to absorb 5 μL of the sample to be tested on each piece of filter paper, the negative control is dimethyl sulfoxide (DMSO), and each sample is repeated three times. Finally, culture the petri dish overnight, measure the size of the inhibition zone with a vernier caliper, and make statistics in Table 1 below.
[0038] Table 1 The inhibition zone experiment of fusidic acid and dihydrofusidic acid on 7 kinds of bacteri
Embodiment 3
[0042] Minimum Inhibitory Concentration and Minimum Inhibitory Concentration Test: The lowest bactericidal concentration and minimum inhibitory concentration of the compound were tested through a 96-well plate. First, multiple dilution gradients of each sample were obtained by the double dilution method, and different Take 5 μL of the concentration sample and add it into the microwell plate with a pipette; secondly, prepare a certain concentration of bacterial suspension (1.5×10 5 CFU / mL) for activity test research, and 195 μL of bacterial suspension was added to the microwell plate using a discharge gun, in which pure liquid medium was selected as the negative control; finally, the microplate was covered and placed in a bacterial incubator to cultivate Overnight, and the absorbance of each well of the microplate was tested by a multi-functional microplate reader at a wavelength of 600nm, and statistics were made to obtain the minimum inhibitory concentration and minimum bacteri
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap