Glucose derivative lactone-cyclic lactone polymer and preparation method thereof
A glucose derivative and cyclic lactone technology, which is applied in the field of glucose derivative lactone-cyclic lactone polymer and its preparation, can solve problems such as unfavorable industrial production, cumbersome process, and failure of polymerization reaction, and achieve good biological Compatibility, expanded application range, excellent non-toxic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
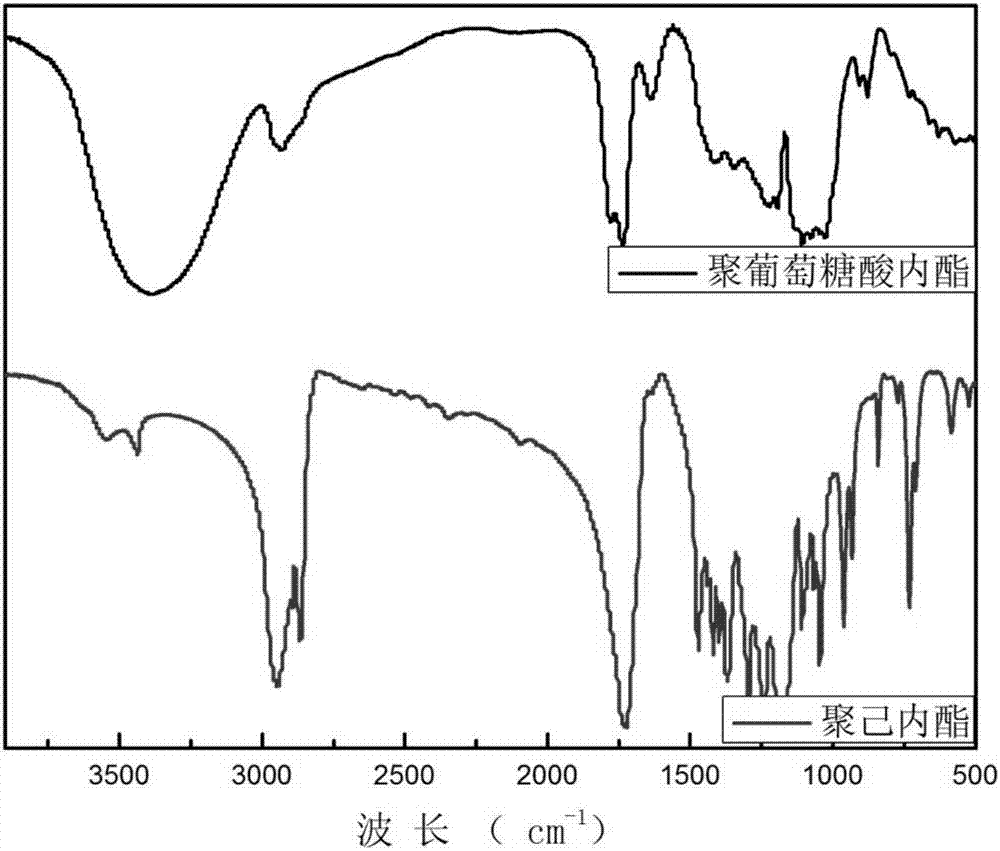
[0034] The reaction process is: under a nitrogen atmosphere, add 49.87mL (0.5mol) of caprolactone and 0.15mL of stannous octoate / toluene solution into the flask, heat up to 130°C, add 0.17mL of diethylene glycol, and react for 48 After hours, the solvent was removed in vacuo to yield polycaprolactone. The Fourier infrared data of the obtained product are as follows. From the infrared data, it can be seen that the polymerization of caprolactone was successful. FT-IR data are as follows figure 1 Shown: ν(cm -1 ): 3,548.60, 2946.05, 2866.16, 1724.31.
Embodiment 2
[0035] Embodiment 2 Preparation of glucose derivative lactone-cyclic lactone polymer of the present invention
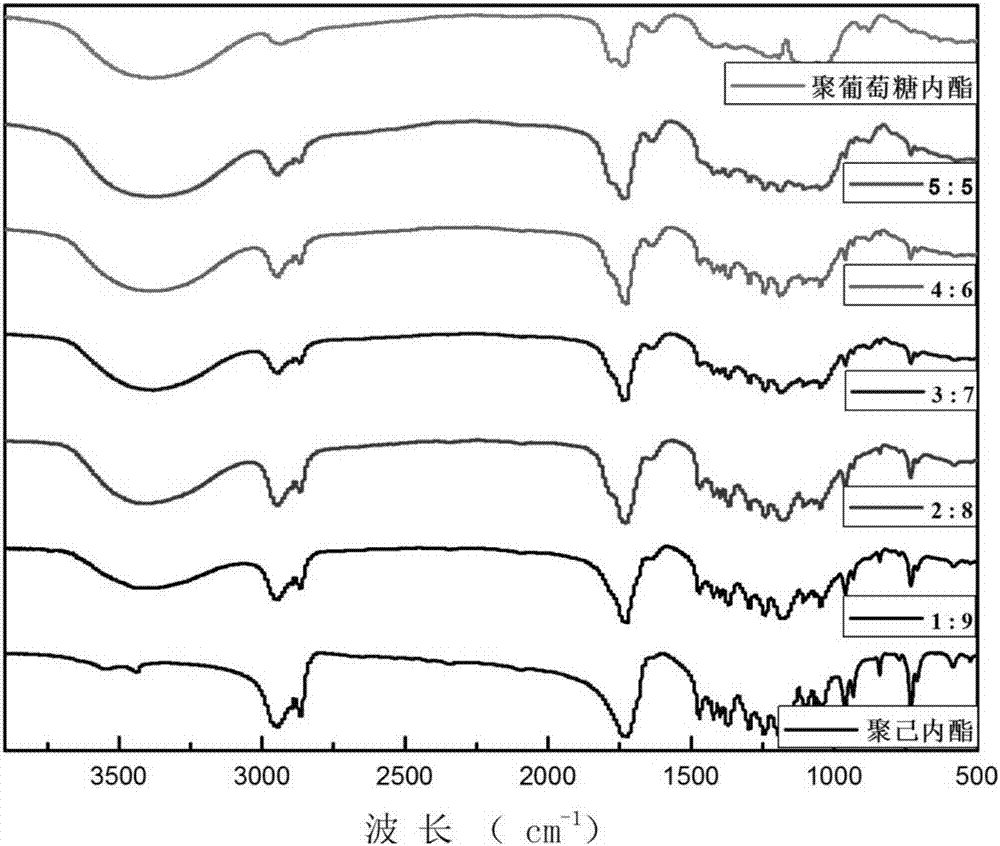
[0036] The reaction process is: under a nitrogen atmosphere, 49.87mL (0.45mol) of ε-caprolactone, 8.91g (0.05mol) of gluconolactone (glucolactone: ε-caprolactone = 1:9), 1.7 g (0.008mol), heated up to 140°C, added 0.18mL (1.8mmol) of diethylene glycol, and reacted for 24 hours to obtain a copolymer of gluconolactone and caprolactone. The obtained product can be seen from the infrared data that butyl stannic acid catalyzes the successful copolymerization of gluconolactone and caprolactone. FT-IR data are as follows figure 2 Shown: ν(cm -1 ): 3,436, 3392, 2946, 2867, 1727, 1294-1185.
Embodiment 3
[0037] Embodiment 3 Preparation of glucose derivative lactone-cyclic lactone polymer of the present invention
[0038] The reaction process is: under an argon atmosphere, 44.33mL (0.4mol) of ε-caprolactone, 17.81g (0.1mol) of gluconolactone (gluconolactone: ε-caprolactone = 2:8), butyl stannic acid 2.6g (0.012mol), heated up to 150°C, added 0.15mL (2.69mmol) of ethylene glycol, and reacted for 12 hours to obtain a copolymer of gluconolactone and caprolactone. The obtained product can be seen from the infrared data, butyl stannic acid catalyzed the copolymerization of gluconolactone and caprolactone successfully. FT-IR data are as follows figure 2 Shown: ν(cm -1 ): 3,384, 2946, 2868, 1731, 1294-1186.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap