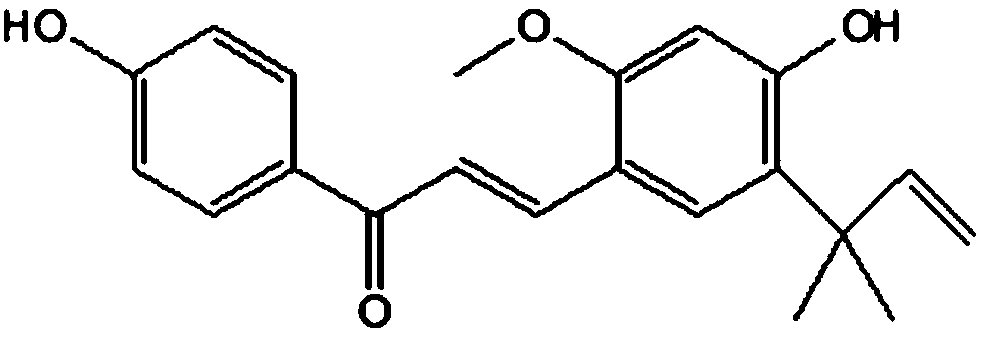
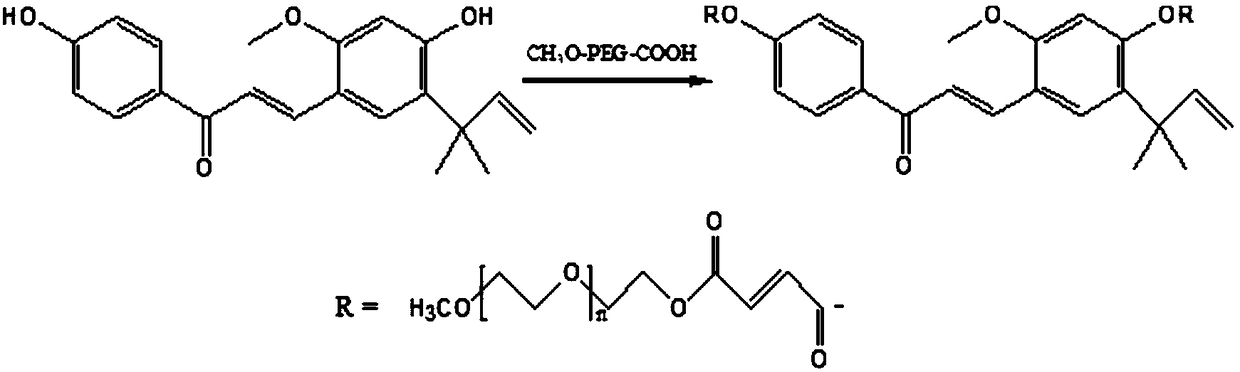
Polyethylene glycol modified licochalcone A preparation method
A technology of licochalcone and polyethylene glycol, which is applied in the field of preparation of licochalcone A, and achieves the effects of mild conditions, simple operation, and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
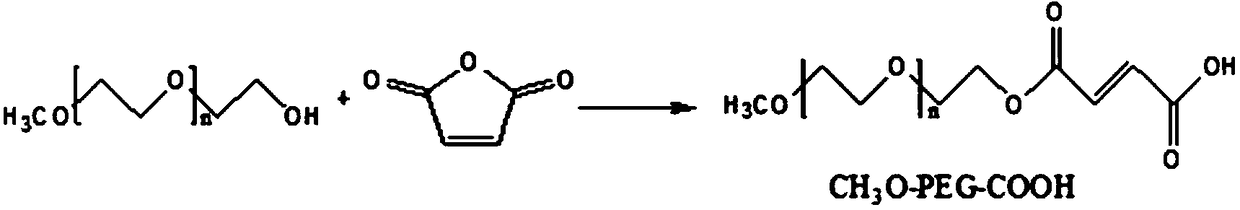
[0047] Step 1: Monocarboxy-terminated polyethylene glycol (CH 3 O-PEG-COOH) preparation, its reaction mechanism is:
[0048]
[0049] Specific steps are as follows:
[0050] Weigh 4mmol of methoxypolyethylene glycol with a molecular weight of 2000, 4mmol of maleic anhydride and DMF into the reaction flask, start stirring, heat the temperature to 60°C, blow nitrogen, and add 2mL of pyridine after the solid is completely dissolved. , control the temperature at 60°C, react for 12 hours, monitor the reaction end point by thin-layer chromatography, concentrate the organic solvent under reduced pressure, drop the concentrated solution into glacial ether for precipitation, wash the solid twice with ether, and dry the solid in vacuum to obtain a yellow solid powder CH 3 O-PEG-COOH.
[0051]Monocarboxyl-terminated polyethylene glycol (CH) provided by the present invention 3 O-PEG-COOH) preparation method, the obtained solid powder CH 3 The spectrum data of O-PEG-COOH is:
[0052]
Embodiment 2
[0060] Step 1: Monocarboxy-terminated polyethylene glycol (CH 3 O-PEG-COOH) Preparation
[0061] Weigh 4mmol of methoxypolyethylene glycol with a molecular weight of 2000, 20mmol of maleic anhydride and DMF, add them into the reaction flask, start stirring, heat the temperature to 75°C, blow nitrogen, and after the solid is completely dissolved, add 2mL of pyridine , control the temperature at 75°C, react for 10 h, monitor the end point of the reaction with thin-layer chromatography, concentrate the organic solvent under reduced pressure, drop the concentrated solution into glacial ether for precipitation, wash the solid twice with ether, and dry the solid in vacuum to obtain a yellow solid powder CH 3 O-PEG-COOH.
[0062] Monocarboxyl-terminated polyethylene glycol (CH) provided by the present invention 3 O-PEG-COOH) preparation method, the obtained solid powder CH 3 The spectrum data of O-PEG-COOH is:
[0063] 1 H-NMR (DMSO-d 6 / ppm): δ3.45-3.62(m,-OCH 2 CH 2 -),3.69(
Embodiment 3
[0069] Step 1: Monocarboxy-terminated polyethylene glycol (CH 3 O-PEG-COOH) Preparation
[0070] Weigh 4mmol of methoxypolyethylene glycol with a molecular weight of 2000, 40mmol of maleic anhydride and DMF, add them into the reaction flask, start stirring, heat the temperature to 80°C, blow nitrogen, and after the solid is completely dissolved, add 2mL of pyridine , control the temperature at 80°C, react for 6 hours, monitor the end point of the reaction with thin-layer chromatography, concentrate the organic solvent under reduced pressure, drop the concentrated solution into glacial ether for precipitation, wash the solid twice with ether, and dry the solid in vacuum to obtain a yellow solid powder CH 3 O-PEG-COOH.
[0071] Monocarboxyl-terminated polyethylene glycol (CH) provided by the present invention 3 O-PEG-COOH) preparation method, the obtained solid powder CH 3 The spectrum data of O-PEG-COOH is:
[0072] 1 H-NMR (DMSO-d 6 / ppm): δ3.43-3.63(m,-OCH 2 CH 2 -),3.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap